Battery Technology
Home » Battery Technology
Today’s advanced batteries require a range of specialized analytical tools to better understand the electrochemical processes that occur during battery cycling. EAG Laboratories offers a wide-range of battery materials characterization services specifically for the battery industry to help with manufacturing, materials R&D and failure analysis.
RAW MATERIALS
During battery manufacture, an important factor affecting performance, and potentially safety, is the consistency in composition and impurity levels in the raw electrode materials. A number of different analytical techniques are available to address this. ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry) is commonly used for analyzing the composition of electrodes, whereas ICP-MS (Inductively Coupled Plasma-Mass Spectrometry) is used for accurately determining lower level elemental impurities.
GDMS (Glow Discharge Mass Spectrometry) allows full periodic table trace element analysis in a single measurement and is an ideal technique for monitoring the presence of unwanted impurities. IGA (Instrumental Gas Analysis) is the technique of choice when analyzing for low levels of gas forming elements such as H, C, N, O and S.
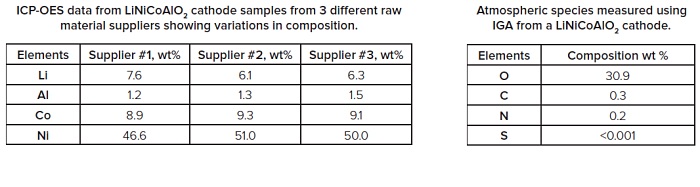
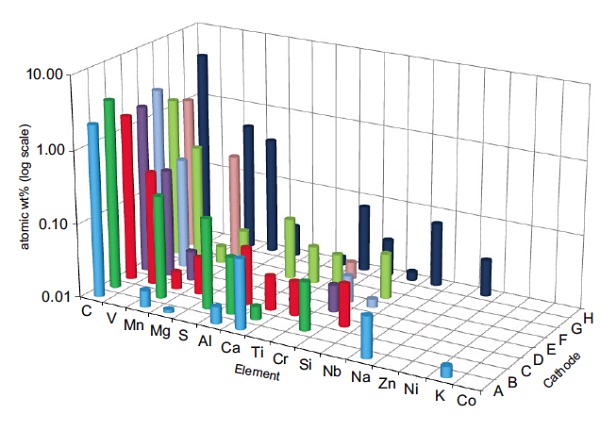
Figure 1 plot shows GDMS data acquired from eight LiFePO4 cathode samples from a range of suppliers. Only selected elements are shown here for clarity. The presence of a number of different unwanted impurities was repeatable over multiple samples from a given batch and provided valuable information to the battery manufacturer. One supplier’s batch had unusually high amounts of impurities, in particular Mn and Mg, and the batteries manufactured from this batch resulted in unacceptable cycle life.
SURFACE CHEMISTRY & COMPOSITION
With increasing demands for higher battery performance and improved safety, a better understanding of the factors affecting performance, cycle life and possible failure mechanisms is essential.
Measuring the chemical state of the battery components such as the cathode, anode, separator, electrolyte, contact layers and additives, at various stages of cycling, provides vital information about the electrochemical processes that occur during battery use. EAG offers elemental and molecular analyses using a number of different analytical tools to address this need. Moisture sensitive materials are extracted from cells in a moisture controlled environment and can be transferred to instruments for analysis without exposure to air or moisture.
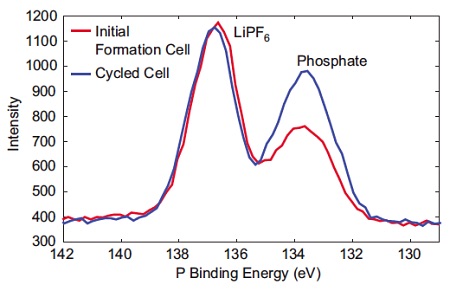
Figure 2 shows XPS (X-Ray Photoelectron Spectroscopy) is a commonly used technique for investigating the surface chemistry of electrodes. Here, the phosphorus chemistry on a graphitic anode before and after battery cycling is compared. After cycling there is a clear increase in phosphate bonding relative to LiPF6.
Figure 3 shows TOF-SIMS (Time-of-Flight Secondary Ion Mass Spectrometry) has the unique ability of analyzing both organic and inorganic components and allows a full investigation of decomposition products; the presence of impurities; or any other surface changes during cycling.
This spectrum was acquired from a cycled battery with a LiCoO2 cathode using LiPF6 electrolyte. A number of molecular species of interest are identified on the surface of the cathode that did not originate there.
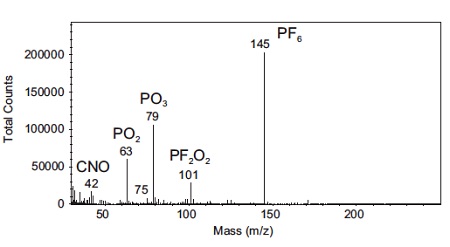
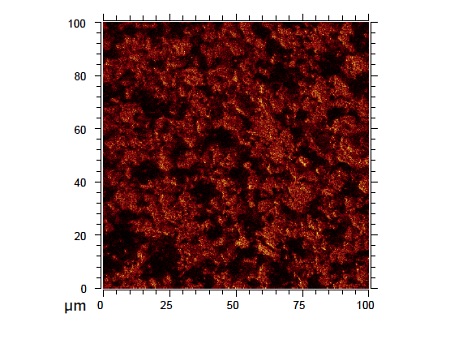
Figure 4 shows This is a secondary ion image recorded from the surface of a lithium titanate (Li4Ti5O12) anode. By imaging different species on the sample, TOF-SIMS allows a better understanding of the lateral chemical distribution of species of interest at various stages of cycling.
Sputtering with an ion beam also allows species of interest to be depth profiled, which is particularly useful in studying the chemical composition of SEI layers as a function of depth.
Figure 5 shows Auger Electron Spectroscopy allows elemental analysis using an electron beam focused down to ~10-20 nm, enabling specific regions of interest on electrode particles to be analyzed.
Here, the surface of a cycled graphitic anode is analyzed showing the presence of species characteristic of both the anode and the electrolyte.
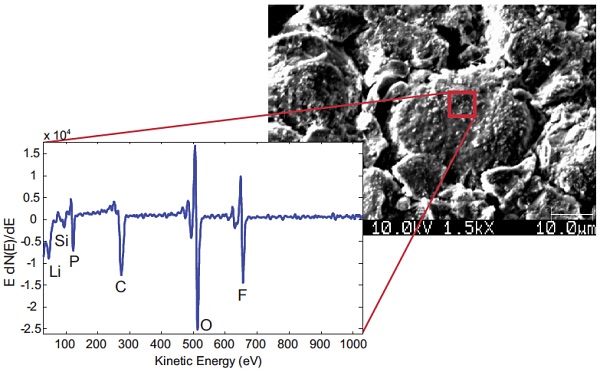
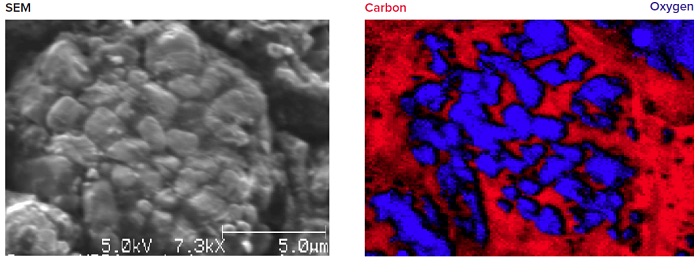
Figure 6 shows using the elemental mapping capability of Auger Electron Spectroscopy (AES), a cycled LiNiMnCoO2 cathode is analyzed showing a high carbon signal at the extreme surface, from electrolyte residue surrounding the cathode particles.
MORPHOLOGY & UNIFORMITY
Electron microscopy techniques such as SEM (Scanning Electron Microscopy), TEM (Transmission Electron Microscopy), DB-FIB (Dual Beam Focused Ion Beam) imaging are essential techniques to investigate morphology, particle size, particle coatings, mixing efficiency and defects. These techniques often employ elemental mapping capabilities such as EELS (Electron Energy Loss Spectroscopy) and EDS (Energy Dispersive X-Ray Spectroscopy) which can provide further valuable information about elemental composition and location/distribution.

Figure 7 shows a secondary ion image recorded from the surface of a lithium titanate (Li4Ti5O12) anode. By imaging different species on the sample, TOF-SIMS allows a better understanding of the lateral chemical distribution of species of interest at various stages of cycling.
Sputtering with an ion beam also allows species of interest to be depth profiled, which is particularly useful in studying the chemical composition of SEI layers as a function of depth.
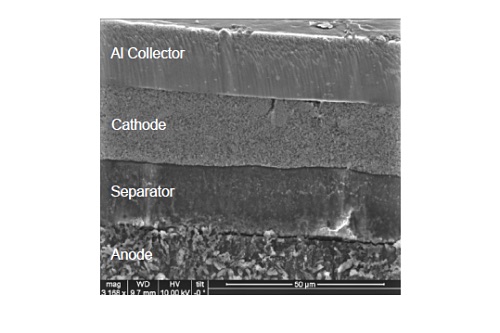
Figure 8 shows an example of an SEM image of a cycled lithium polymer battery prepared by ion milling. Film thickness variation and imperfections at the interfaces can be easily inspected. Each layer can be further inspected at greater magnification.
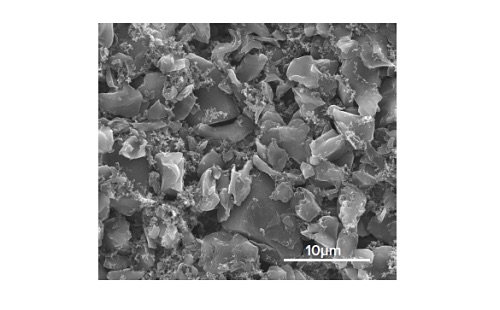
Figure 9 shows an SEM image of a freshly prepared carbon based anode film. Variation in particle size and mixing efficiency can easily be investigated. Software can be used for further image processing to aid in this task.
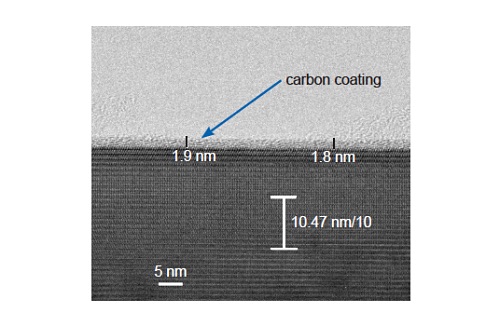
Figure 10 shows a TEM image of a freshly prepared cathode shows an amorphous carbon coating on a LiFePO4 particle. The thickness can be accurately measured, allowing thickness variation to be monitored. The elemental composition of the coating can be confirmed by EELS.
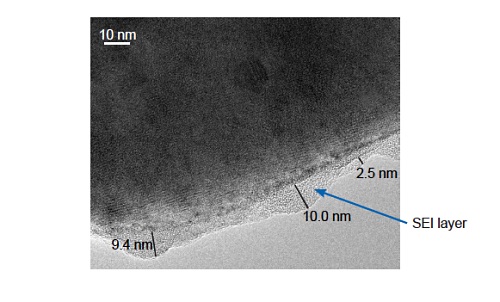
Figure 11 shows a TEM image of a cycled LiFePO4 particle showing the formation of an SEI (solid-electrolyte interface) layer on the surface. Determining the composition of SEI layers via EELS or EDS can provide important insights into possible SEI growth mechanisms.
Battery Materials Characterization Services
Typical battery services include:
- Composition
- Impurity Surveys
- Phase Identification
- Chemical Analysis
- Electron Microscopy
- Thermal Stability
- Elemental Analysis
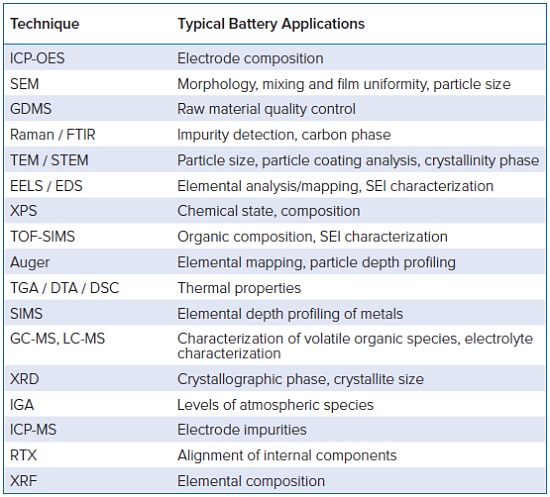
Battery Materials Characterization Techniques
Would you like to learn more about Battery Technology?
Contact us today for your battery technology needs. Please complete the form below to have an EAG expert contact you.