In Vitro Biocompatibility
Home » Services » Materials Testing & Analysis » In Vitro Biocompatibility
The Eurofins EAG In Vitro Biocompatibility Laboratory offers a comprehensive battery of biocompatibility and consumer product safety testing services.
In vitro biocompatibility analysis is a set of methods used to identify potential health hazards from a sample without the use of in vivo animal testing. The results of biological models in response to test samples help indicate the physiological outcomes that could occur during the real-world use of the product.
Traditional models that rely heavily on animal testing face ethical and stringent regulatory challenges. In vitro models offer a more humane, and cost-effective alternative. Thorough in vitro testing can help identify potential safety hazards during the earliest stages of product development before proceeding to more expensive and time-consuming in vivo studies or clinical trials.
Benefits of In Vitro Testing at EAG
From R&D design screening to lot release and regulatory submission, manufacturers and suppliers are looking for in vitro testing that provides materials expertise with quick turnaround.
At EAG, our scientists approach in vitro testing from a different direction. As materials experts, we understand the technical aspects of the assays and the chemistry of materials. This allows us to offer a greater understanding of why a certain material failed an assay.

In Vitro Testing at EAG
- Cytotoxicity
- MEM Elution
- Direct Contact
- Agar Diffusion
- Growth Inhibition IC50
- Skin irritation
- EpiDerm™ RhE model
- Skin sensitization
- Genotoxicity
- Micronucleus Assay (OECD 487)
- Ames Bacterial Reverse Mutation Assay (OECD 471)
- Custom Assays
High throughput for in vitro testing is important. But when a material fails an assay, understanding why is crucial. The EAG team expanded our In Vitro Biocompatibility Lab to meet these client needs. Reach out to our team for help with your project.
Ideal Uses of In Vitro Testing
-
Biocompatibility testing for regulatory submission
-
Research & development of products and materials
-
Impact of manufacturing and cleaning processes
-
Design change testing
-
Safety testing for consumer products and wearable technology
-
Raw material screening
-
Lot release testing
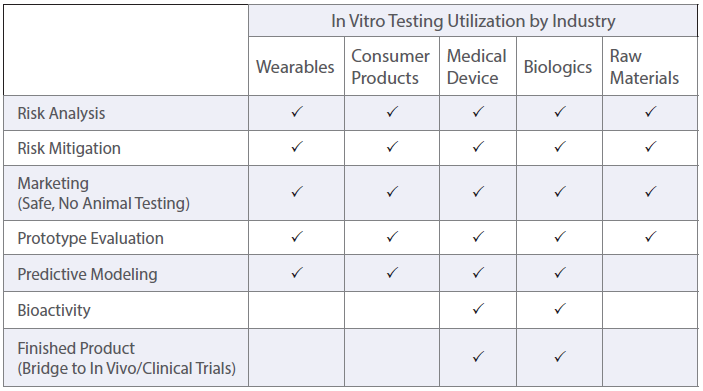
All experiments are designed to adhere to common industry and regulatory standards. Several endpoints can be evaluated using in vitro techniques, including cytotoxicity and irritation. Further, each endpoint can have several methods of analysis that provide different details on the extent of the result.
This testing allows for flexibility and analysis that is specific to the sample’s anticipated usage in a controlled environment. Visit our blog to learn more about the principles and strengths of In Vitro Testing.
Would you like to learn more about In Vitro Biocompatibility Testing?
Contact us today for your In Vitro Biocompatibility testing needs. Please complete the form below to have an EAG expert contact you.
