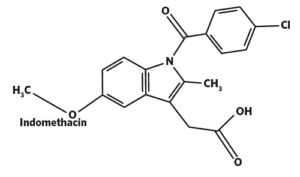
Quantification of Active Pharmaceutical Ingredients by XPS
API Quantification on the Surface of Pharmaceuticals can be done by X-ray Photoelectron Spectroscopy (XPS) from EAG Laboratories.
Eurofins EAG Laboratories scientists have conducted pharmaceutical testing with virtually every class and type of compound across most indications and all common formulations of pharmaceuticals, biopharmaceuticals and generic drugs. We have helped hundreds of companies overcome R&D roadblocks, defend their patents, respond to regulators and uncover sources of problematic manufacturing issues.
How do I ensure the proposed study will adequately inform my next development decision? How do I know what E&L data the FDA will require? How can I ensure supplier changes don’t impact quality? For more than two decades, EAG scientists have been helping the pharmaceutical industry solve problems like these. Through multidisciplinary science and engineering, we improve product development efficiency and expedite time-to-to market.
EAG’s pharmaceutical testing services are customized according to each individual project’s needs. We apply single or multiple methods of analysis to test chemicals and their raw materials. This could be as simple as confirming the identity of a material, to more complex scenarios where the presence of additives or impurities in a material is of interest.
Turn to EAG. WE KNOW HOW.
EAG scientists support new product development, reformulation and lifecycle initiatives with primary research and exploratory studies, a broad array of GLP and cGMP-compliant analytical services, and drug and device development expertise. Whether we’re developing a complex analytical method or cell-based bioassay, characterizing intermediate materials or performing routine sample testing, you can count on EAG to deliver well-thought out study designs, reliable data, expert interpretation and rigorous regulatory compliance.
When things don’t go as expected, it is critical to have solid science on your side, and the response must be specific and disciplined. EAG has decades of experience investigating issues related to supply chain inputs, process and product failures, packaging and other sources of contamination. Our scientists are experts at developing innovative test designs to troubleshoot problems across all major dosage forms and delivery systems—from coated tablets to unit dose vials (UDVs) and metered dose inhalers (MDIs). EAG offers deep expertise in the isolation, elucidation, identification and characterization of trace levels of knowns and unknowns for both regulated and non-regulated studies.
EAG supports quality assurance and quality control initiatives with independent stability and release testing, certificates of analysis for drug and drug product, and third party verification for supply chain inputs, components and packaging. From compliance with the latest USP <232/2232> guidance on trace elemental analysis to routine stability and QC release testing, EAG is here for you.
EAG scientists have the experience required to translate guidelines into study designs that deliver the specific, reliable data that regulators expect. This not only helps you avoid unexpected delays and related costs, it helps to efficiently progress programs to their next development milestone. All regulated studies are conducted under strict compliance with Good Laboratory Practice or Current Good Manufacturing Practice guidelines. EAG has a stellar FDA inspection history, including an unblemished track record for successful FDA pre-approval inspections.
EAG offers comprehensive CMC analytical support and quality control for API and drug product for all dosage forms, performed under phase-appropriate CGMP. Areas of particular expertise include extractables and leachables programs, container-closure systems selection and qualification, and the development, validation and analysis of process, product and host cell protein impurities to support biopharmaceutical stability programs, forced degradation studies, and product and process investigations.
EAG’s experience extends beyond the laboratory and into the court room. We maintain a team of litigation experts who regularly assist clients with trial preparation, depositions and expert witness testimony. EAG scientists have extensive experience supporting cases involving intellectual property, product liability and insurance claims, as well as evaluating the scientific validity of academic publications, media reports and other claims for government institutions and private industry.
From formulation analyses to contaminant and packaging investigations, EAG scientists support every phase of the drug product lifecycle.
We offer comprehensive analysis of layer structure, chemical composition, particle size and imaging of a drug. This includes quantitation and distribution of active ingredients, excipients and coatings at the sub-micron scale.
Also known as reverse engineering, deformulation is the separation, identification and quantitation of ingredients in a product. This can be useful when you are developing a generic equivalent or defending your own IP.
Biosimilars services include peptide mapping, post-translational modification (PTM) identification, intact analysis, drug antibody ratio (DAR) calculations for antibody drug conjugates (ADCs), and expert witness services.

EAG brings multi-disciplinary expertise to the isolation, elucidation, identification and characterization of trace levels of knowns and unknowns, including contaminants in packaging, ingredients and final products.
From reference standards, test articles to stable-labeled active ingredients we offer multi-step and other complex synthesis projects. EAG’s analytical services ensure quick turnaround of purity and structural confirmation.
EAG maintains a team of scientists and engineers who provide technical consulting, problem solving and litigation support, including expert testimony for patent violation investigations, product liability and other legal issues.
EAG is well known for investigative problem-solving. Whether you’re dealing with a black speck, off color, packaging failure or some other problem, our troubleshooting experts have a stellar track record for identifying root cause quickly. For other preclinical, clinical and CMC development services, we partner with our colleagues at Eurofins Biopharmaceutical Product Testing. For environmental risk assessments, we work with Eurofins Regulatory Science Services.
Download the Brochure Today!
Contact us today for your pharmaceutical testing needs. Please complete the form below to have an EAG expert contact you.

API Quantification on the Surface of Pharmaceuticals can be done by X-ray Photoelectron Spectroscopy (XPS) from EAG Laboratories.

SEM-EDS and RAMAN analytical testing laboratory techniques applied to identification of foreign material contamination on pharmaceuticals.
ICP-MS is a multi-elemental bulk chemical analysis technique that can determine simultaneously up to 70 elements in a single sample.
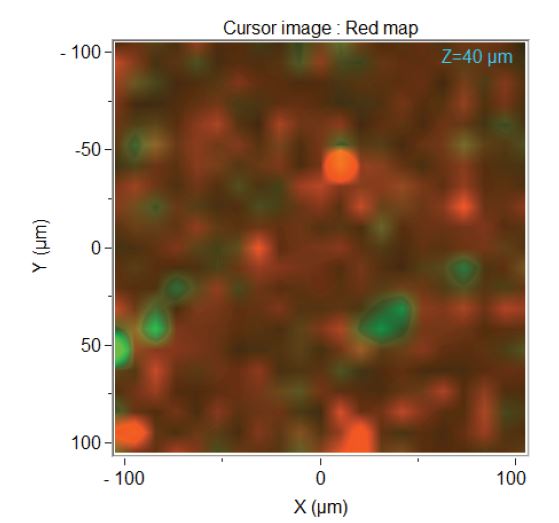
A very good use of Raman is for the analysis of pharmaceutical products to determine the spatial distribution of components of interest
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.