
Extraction Expertise for Medical Device Chemical Characterization
EAG offers advanced expertise and a risk-based approach for medical device extractable study design and execution, including the latest FDA expectations.
Home » Using ICP-MS to Measure Elemental Compositions in Drug Products
The Food and Drug Administration (FDA) is a regulating body whose mission is to protect public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices.1 When creating new drugs to bring to market, pharmaceutical companies are governed by regulations to ensure consumer safety. Factoring in costs of production and research, companies want to make sure their product is safe and will pass all inspections when submitted to the FDA. For example, manufacturers must meet United States Pharmacopeia (USP) chapters <232> Elemental Impurities-Limits and <233> Elemental Impurities-Procedures that specify limits and procedures for elemental impurities in drug products. One instrumental technique used to test for these impurities is Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
Elemental impurities, specifically heavy metals, can be introduced from various sources during the different phases of manufacturing
pharmaceutical products. They can derive from raw materials, active pharmaceutical ingredients (APIs), formulation agents, solvents, reactors, catalysts, transferring pipelines, and other equipment used during production. The United States Pharmacopeia (USP) identified and set limits on the concentration of several elements in pharmaceuticals and categorized them into three classes based upon their toxicity and chance of detection in the final drug product. The Class 1 elements include As (arsenic), Hg (mercury), Cd (cadmium), and Pb (lead); also known as the “Big Four”, these elements are known or strongly suspected human toxicants and thus should be essentially absent (<1 μg/day). The Class 2 elements, Co (Cobalt), V (vanadium), Ni (nickel), Tl (thallium), Au (gold), Pd( palladium), Ir (iridium), Os (osmium), Rh (rhodium), Ru (ruthenium), Se (selenium), Ag (silver), Pt (platinum) , along with Class 3 elements Li (lithium), Sb (antimony), Ba (barium), Mo (molybdenum), Cu (copper), Sn (tin), Cr (chromium) are slightly less toxic than the Big Four, though still run the risk of appearing in final drug formulations. These impurities can also affect the stability and shelf life of drugs because of their catalytic activities. This is true for oral drugs (e.g., vitamins), inhalation drug products (e.g., bronchodilators to alleviate asthma), and topical formulations (any product applied to the skin).2
ICP-MS is a multi-elemental technique with extremely high sensitivity and large linear dynamic range. This allows simultaneous analysis of main components and ultra-trace elements. ICP-MS is a bulk chemical analysis technique that can determine simultaneously up to 70

elements in a single sample analysis. It can analyze elements from Li to U and can be applied to solutions and digested solids. Instrumentation is also suitable for automation thus enhancing accuracy, precision, and throughput.
ICP-MS has been the technique of choice since 2018, when the previous method, USP 231 was officially replaced with the new monograph. Previously, elemental impurities were determined in pharmaceuticals using qualitative methods based on impurities forming colored complexes with the sulfate ion3. This technique could not distinguish exactly which heavy metals were present – just that some were there. Furthermore, some regulated elemental impurities do not reliably form sulfate complexes and can escape detection entirely. The use of ICP techniques remedied these limitations due to their ability to unequivocally measure the target elements under test based upon the more rigorous criteria assigned in USP 233.
The scientists at EAG are experts at utilizing ICP-MS to determine which elements are present and at what concentration. They are experienced in working with clients on validation studies for submission to the FDA. Contact us today to learn more about our capabilities for measuring elemental impurities in pharmaceuticals.
2Kwan H. Nam, Robert Isensee, Gabe Infantino, Karol Putyera, and Xinwei Wang. “Microwave-Induced Combustion for ICP-MS: A Generic Approach to Trace Elemental Analyses of Pharmaceutical Products.” Spectroscopy Online 1 April 2011: 6. 14 June 2022. <https://www.spectroscopyonline.com/view/microwave-induced-combustion-icp-ms-generic-approach-trace-elemental-analyses-pharmaceutical-product>.
1U.S. Food and Drug Administration. 3 28 2018. <https://www.fda.gov/about-fda/what-we-do>.

EAG offers advanced expertise and a risk-based approach for medical device extractable study design and execution, including the latest FDA expectations.

Televisions, computer screens, cell phone screens etc. are getting clearer, sharper, brighter and use much less power. This is all possible through LEDs.
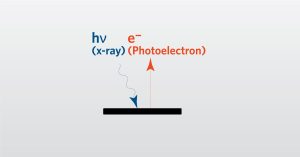
In this webinar we introduce X-Ray Photoelectron Spectroscopy (XPS) which is a surface analysis technique.

FA plays a critical role in product development and improvement. During this live Ask the Expert event, we will answer pre-submitted questions from our audience about electronic device failure analysis.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.