SEM-EDS Webinar
In this webinar we will focus on Energy Dispersive X-ray Spectroscopy (EDS) and Scanning Electron Microscopy (SEM)
Home » Extraction Expertise for Medical Device Chemical Characterization
Extractable studies are performed on medical devices to evaluate potential chemical release from a medical device during clinical use.
The primary guidance for medical device extractable studies is ISO 10993-18 “Chemical characterization of medical device materials within a risk management process”. In this international standard, a number of extraction study approaches are laid out, which are applied to medical devices based on the intended device use.
EAG offers advanced expertise and a risk-based approach for medical device extractable study design and execution, including the latest FDA expectations.

ISO 10993-18 provides guidance for the type of extraction study necessary for chemical characterization, based on device contact.


In this webinar we will focus on Energy Dispersive X-ray Spectroscopy (EDS) and Scanning Electron Microscopy (SEM)

During this Ask the Expert webinar, our team of battery materials experts will address your pre-submitted questions during the event, as well as present about our new battery lab’s capabilities.

FTIR, Raman and NanoIR are particularly well suited at determining the identity and molecular structure of organic materials, however they can also obtain some inorganic information too.
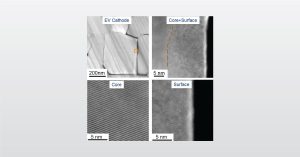
In this webinar we introduce the application of Electron Microscopy to Lithium Ion Batteries from Micron to Atomic Level
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.