EAG Secures DLA MIL-STD 883 TM 1018 Suitability
17th November 2022
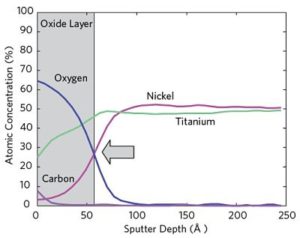
EAG has been approved as a suitable commercial laboratory to provide MIL-STD 883/750 Test Method 1018 Internal Water Vapor Content Testing for hermetically sealed electronic devices.