
Understanding Energy Density in Batteries
There is still so much to learn about batteries, including challenges such as energy density, cycle life, fast charge, and safety. In this blog, we’ll be focusing on energy density.
Home » Principles and Strengths of In Vitro Testing
In vitro is the Latin term for “in glass,” meaning that the testing is performed in a container that is outside of a living organism. This testing uses cell-based biological models instead of animals or humans. In vitro efforts help fulfil the FDA’s “3Rs approach” to replace, reduce, and/or refine animal testing.
Replace
Reduce
Refine
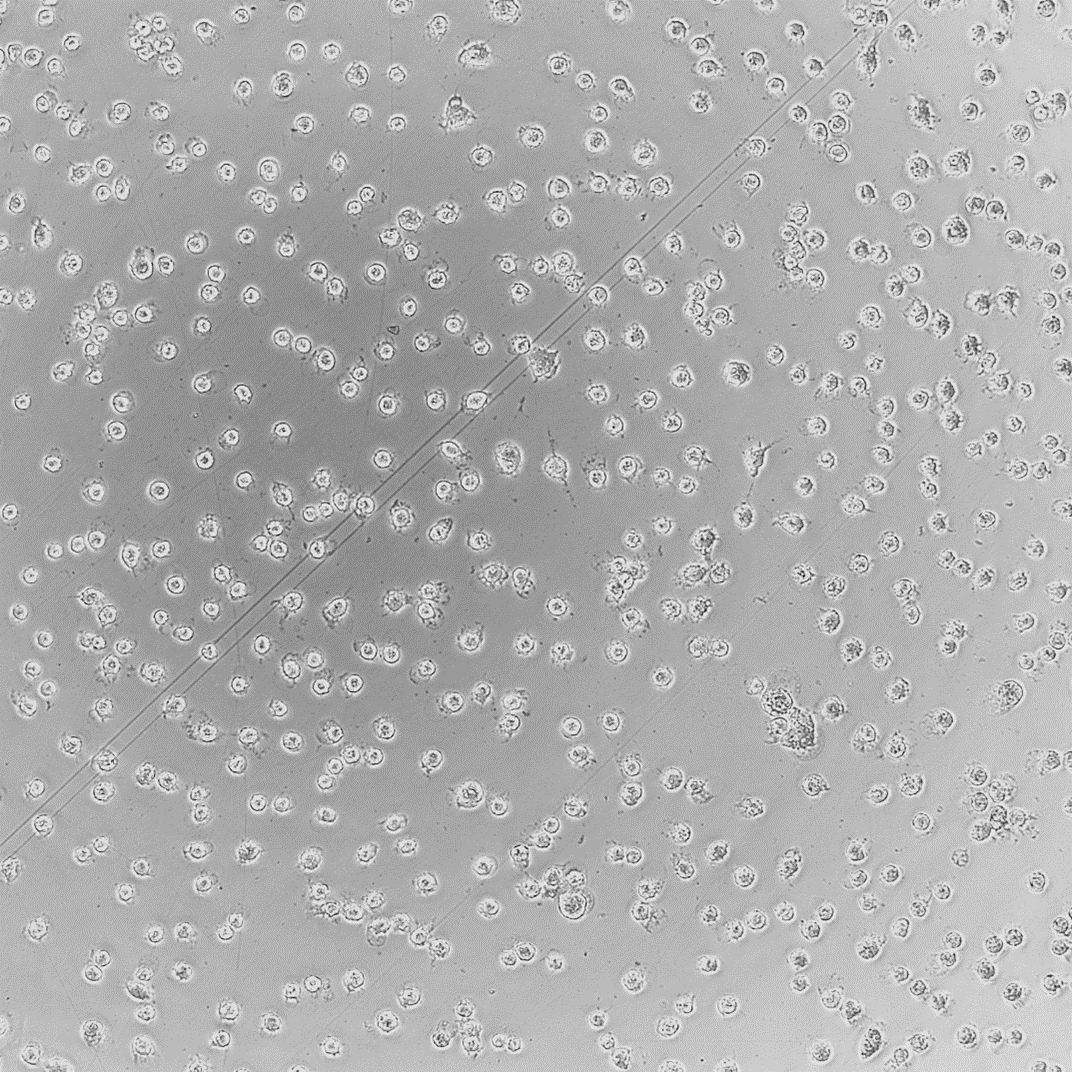
In Vitro model systems can be specified to the test sample’s intended use. 2D cell cultures are grown rapidly for high-throughput or multi-sample analysis with minimized variation between treatments. Cell cultures can also be made into 3D tissue models. These models contain differentiated cell layers to physiologically represent complex tissues and evaluate specific endpoints. Using specific geometries, in vitro cell culture models can be used to evaluate simple and complex biological responses.
To assess different biological endpoints, various forms of analytical methods may be implemented. Measurable responses of the in vitro models include cell death, growth inhibition, genetic alterations, changes in surface marker expression and altered metabolism.


There is still so much to learn about batteries, including challenges such as energy density, cycle life, fast charge, and safety. In this blog, we’ll be focusing on energy density.
February 12, 2025
During this live Ask the Expert event, we will answer pre-submitted questions from our audience regarding Silicon Carbide for High Powered Electronics. EAG Laboratories has a vast depth of experience analyzing silicon carbide using both bulk and spatially resolved analytic techniques and is the world-leading materials characterization and engineering resource for semiconductor testing.

High reliability electronic components like integrated circuits are often required to operate for long periods of time, having little or no opportunity for replacement.
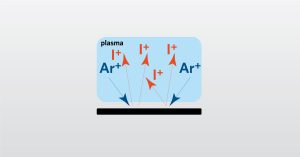
In the full webinar we will focus on full survey chemical analysis using solid sampling high resolution GDMS.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.