
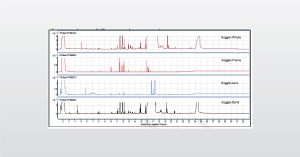
A Deep Dive into Dynamic SIMS vs. static-SIMS
Different types of SIMS analyses exist. The two main types include Dynamic SIMS (high current) and static-SIMS (low current).
Home » Frequently Asked Questions About In Vitro Irritation and Cytotoxicity Testing
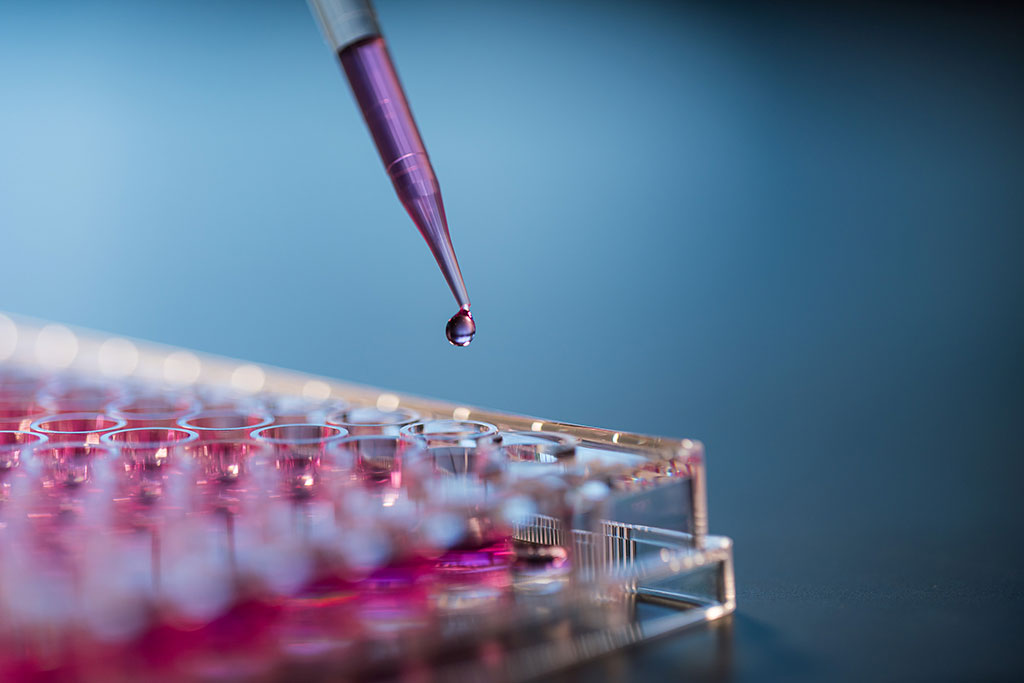
Biocompatibility assessments are a battery of tests performed to evaluate a product, medical device, or other material for the risk of biological hazards. The first test performed is typically a cytotoxicity evaluation. This is done by preparing an extract of the sample to treat a monolayer of healthy mammalian cells in vitro. Results of this assay can indicate the potential toxicity risk a product carries without testing on animals.
Irritation is a localized non-specific inflammatory response to a single, repeated, or continuous application of a substance or material. The in vitro irritation test involves an extraction in physiologically relevant solvents. These extracts are applied to a reconstructed human epidermis model which contains the major layers of cells that are found in a true human epidermis. Death of the cells in this model after exposure to the extracts collected from a product indicate that an irritating substance was leached off the material and poses a risk of adverse reactions in the end user.

Different types of SIMS analyses exist. The two main types include Dynamic SIMS (high current) and static-SIMS (low current).
In this webinar we introduce Extractable and Leachable tests which identify chemical components that can migrate out of a product.

In sectors like the automotive industry, ATE plays a pivotal role in verifying the proper and reliable operation of critical systems.
Contamination control and defect reduction are critical issues in the manufacturing process of compound semiconductor devices which can impact the performance of the end product. We can provide valuable insights to identify contaminants and characterize materials throughout the product lifecycle.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.