Confronting Contamination Analytical Case Studies
Home » Confronting Contamination Analytical Case Studies
Contaminant identification is of great concern to the pharmaceutical and medical device industry. Therefore, it is critical to understand and find a potential contaminant’s origin quickly. Two contaminant identification case studies will be presented. The use of multiple analytical techniques is paramount to determining a contaminant’s identity. These case studies include analytical techniques such as Fourier Transform Infrared Spectroscopy (FT-IR), Scanning Electron Microscopy Energy Dispersive X Ray Spectroscopy (SEM/EDS), and Gas Chromatography Mass Spectrometry (GC/MS).
The first case study is a contaminant identification of a brown material present on a pharmaceutical tablet. This brown material was the source of a potential field recall of an entire lot of a drug product. After isolation and rush analysis, the contaminant was identified, it clearly came from an external source at the consumer end and a field recall was unnecessary for the product.
The second case study presented is material isolated during the cleaning validation of medical grade tubing. The yellow material observed was analyzed by FTIR and SEM/EDS in order to determine the bulk chemical identification. The material was determined to be consistent with polymeric material of the bulk tubing material that was causing issues to downstream processes. After identification, the material was quantified using Gel Permeation Chromatography (GPC). This allows the client to be able to monitor the cleaning procedure.
Case Study #1: Pharmaceutical Tablet with Brown Material
A pharmaceutical tablet was submitted for the analysis of a brown material present. The contaminant was the source of a potential field recall of an entire drug lot.
- The sample was prepared by isolating the brown contamination under a microscope for FTIR analysis.
- Due to the size of the contamination from the tablet, a microscope accessory was utilized for analysis.
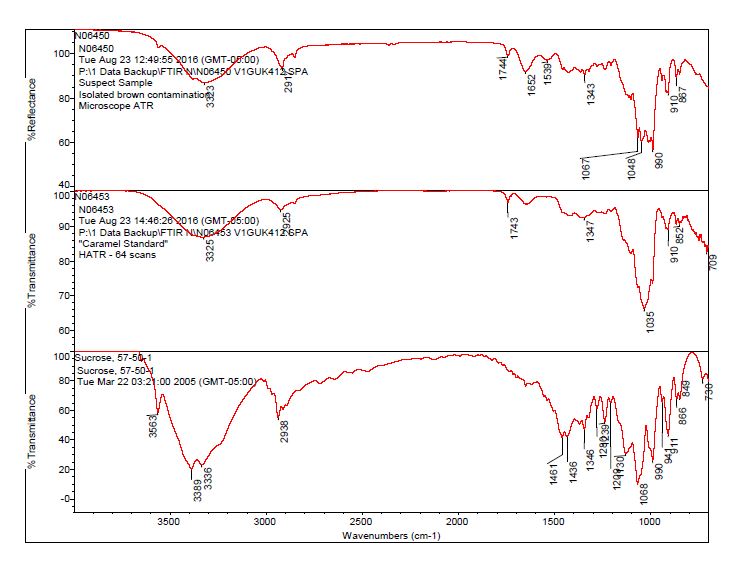
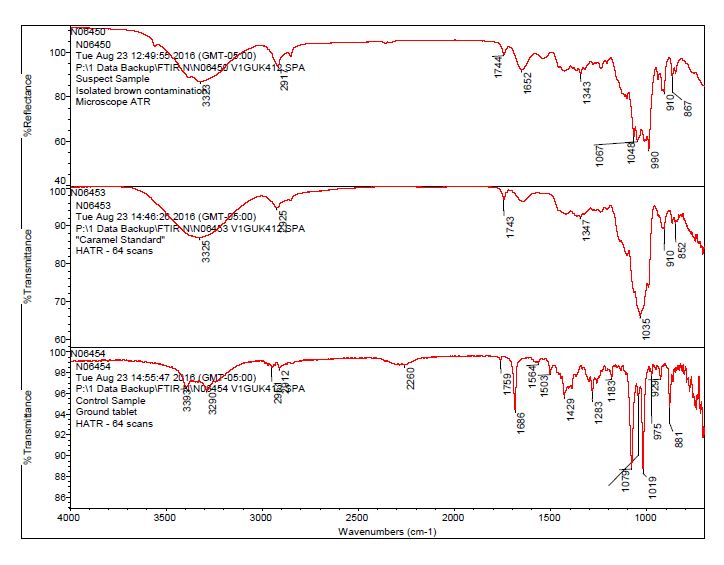
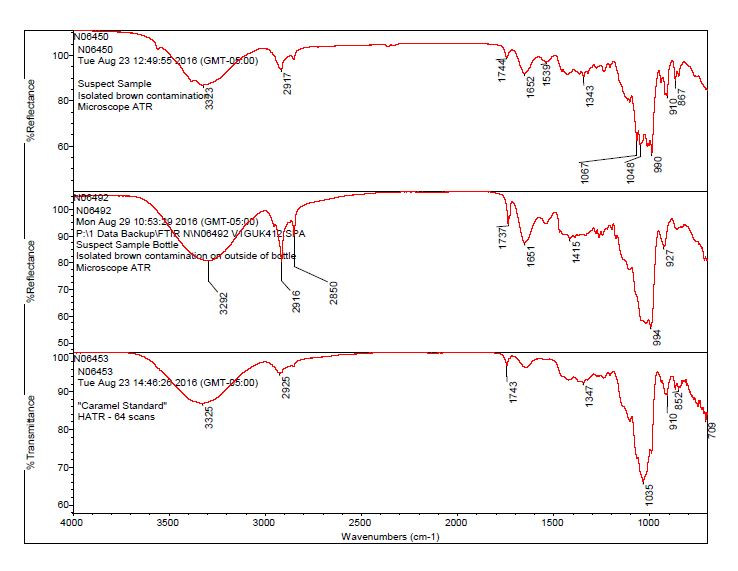
- After observing a library match of sucrose for the contamination, an in-house caramel sample was analyzed to determine the source of the bands 1744 cm-1 and 1652 cm-1.
- The brown residue observed on the outside of the “suspect” bottle was consistent with the brown residue observed on the “suspect” tablets. The data suggests the contaminant on the tablet and bottle is consistent with a sugar material, potentially caramel.
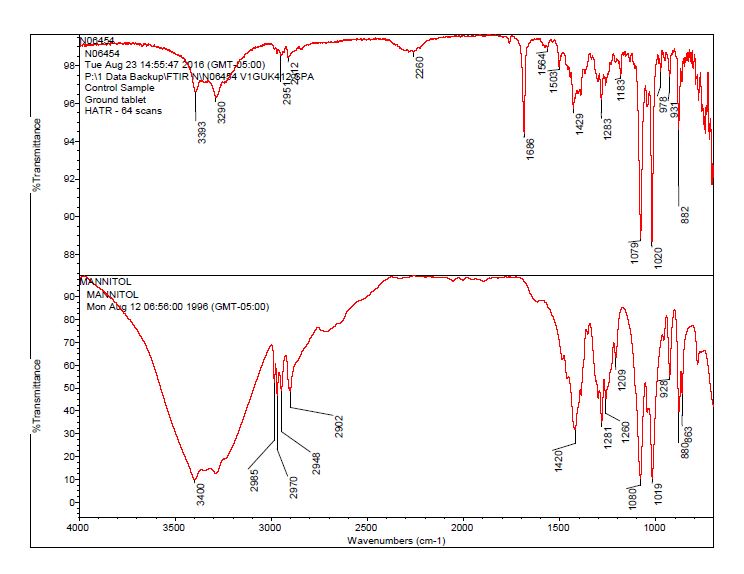
- The bulk material of the tablet was determined to be consistent with mannitol, a known excipient in this tablet. See Figures 1-4 for representative spectra from the analysis.
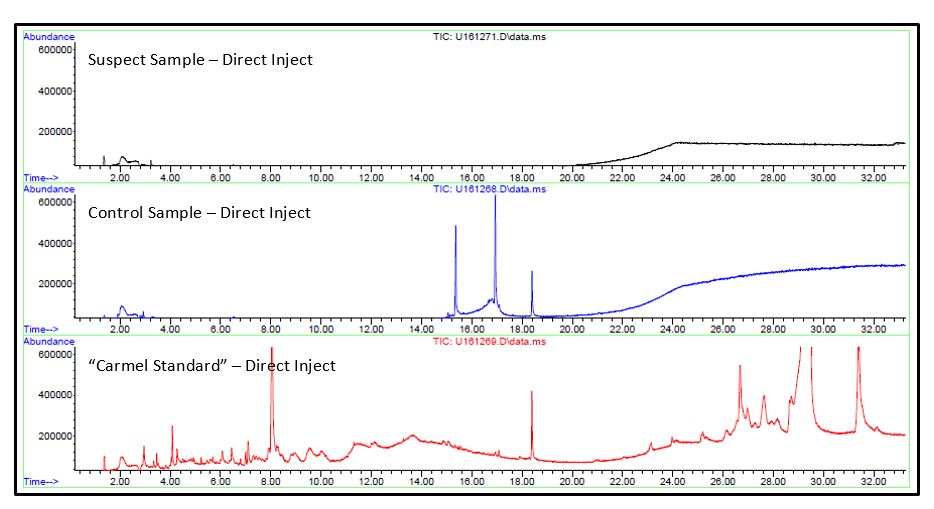
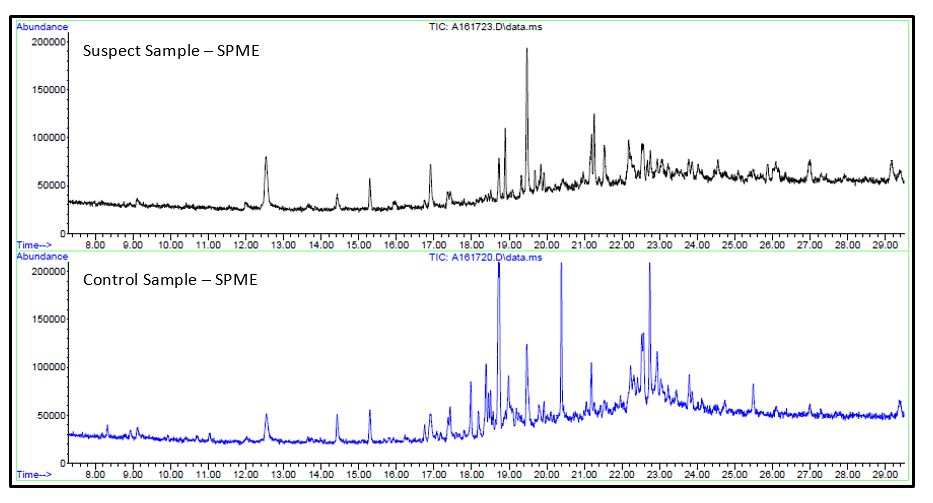
The prepared samples were analyzed by direct inject GC/MS and Solid Phase Microextraction (SPME) GC/MS.
- No significant differences were observed in either analysis between the suspect or control samples. Unique analytes observed in the caramel extracts were identified.
- Reponses for these analytes were searched for in the chromatogram for contaminant, both direct injection and SPME injection, by using extracted ion chromatography (EIC) for unique ions of the caramel components.
- No responses were observed that were consistent with caramel. Since the contamination was a crystalline-like material, a majority of the volatile content may have been lost before analysis.
Case Study #2: Medical Device with Yellow Waxy Material
A yellow waxy material was observed during a cleaning validation procedure. In order to identify the source and nature of the material FTIR and SEM/EDS were performed.
- Under a microscope, yellow particles were physically isolated from the fibrous gauze material on which the sample was submitted.
- These particles were analyzed by microscope FT-IR and SEM/EDS. The FT-IR spectra of the yellow contamination were determined to be consistent with the tubing outer material, an oligomer of the polymer material.
- The SEM/EDS data supported this, with the strongest signals observed being carbon (“C”) and oxygen (“O”) and trace responses from sulfur (“S”) and nitrogen (“N”). Trace responses were also observed for magnesium (“Mg”), aluminum (“Al”), silicon (“Si”), and chlorine (“Cl”).
- The outer surface of the material was not consistent with the inner material.
- It was determined that an oligomer of the polymer may be causing the issue.

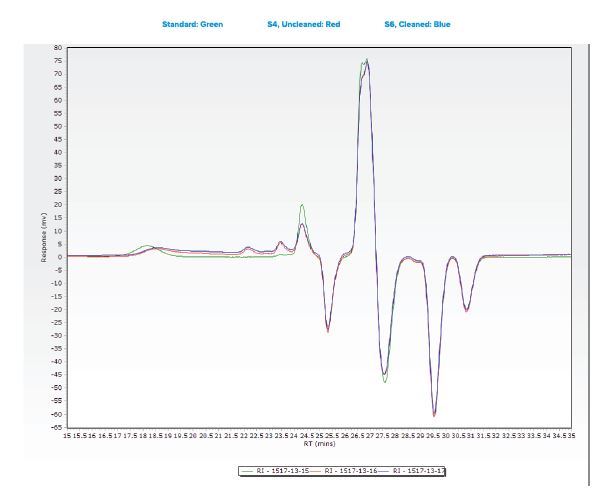
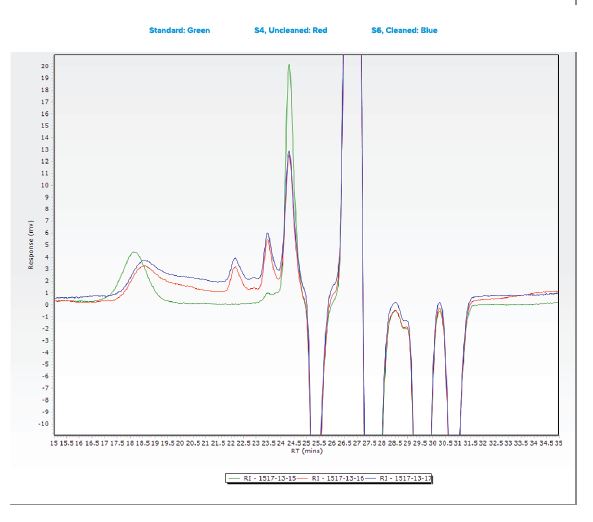
Additional samples were submitted for analysis using Gel Permeation Chromatography (GPC).
- An analyte with a molecular weight of 370 was observed in all three samples.
- This species observed was observed at comparable levels in two submitted samples, indicating that the cleaning process did not effectively remove the material from the tubing.
- Another species was observed in all three samples with a molecular weight between 10,087 and 19,414.
- The large gap in the calculated molecular weight is most likely due to differences in sample matrix when comparing the standard “gauze” extraction to the sample tubing extraction.
Conclusion
Contaminant identification is of great importance in the medical device and pharmaceutical industries. Identifying the source of the contaminant can help save time and money for the manufacturer. We’ve shown in these two case studies that specialized analytical techniques can help identify contamination.
Would you like to learn more about Contaminant identification?
Contact us today for your Contaminant identification needs. Please complete the form below to have an EAG expert contact you.