GMP, GLP or ISO 17025: How Do These Apply to Outsourced Analytical Testing?
Home » GMP, GLP or ISO 17025: How Do These Apply to Outsourced Analytical Testing?
Which medical device regulations apply to my development project?
The regulatory landscape can be complicated for medical device development. The regulations are not entirely clear at times when compliance is required and which standards are needed. Subject to interpretation, this leaves companies to decide how to comply.
- How do you know the appropriate quality standards for outsourcing analytical projects to support medical device product development?
- Should you require a GLP or GMP regulated laboratory?
- What does an ISO 17025 certified analytical laboratory mean?
Contract laboratories must have a robust approach in quality to meet the diverse expectations from their customers. Few contract laboratories have the expertise to guide and consult with their customers and provide recommendations on the correct scientific
study and the proper regulations. The contract laboratory should recognize that the medical device client is tasked with having to know the complexities of bringing a product to market in a highly regulated environment in which quality requirements will change based on the product’s stage in the development cycle.
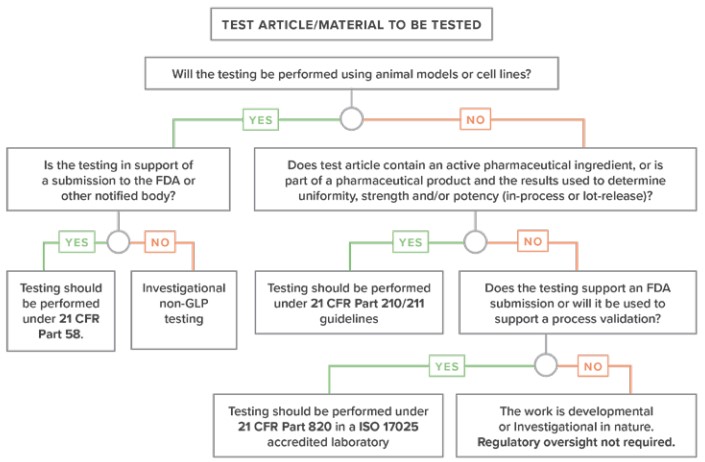
As an analytical testing partner to the medical device industry, it is the laboratory’s responsibility to have a deep understanding of these standards and regulations. Laboratories can help clients not only make the most appropriate choice in the analytical technique and test to solve their problems but also make sure the testing performed will meet the regulatory scrutiny needed to bring safe and effective products to market.
This white paper presents a brief history of medical device regulations, and discusses how the requirements apply to analytical testing of medical devices, including GLPs, GMPs and ISO 17025. A recommended decision tree is presented, to help determine what set of regulatory requirements are needed for medical device testing.
Download our white paper today, and contact us with your questions about your medical device regulations challenges.
Would you like to learn more about GMP, GLP or ISO 17025?
Contact us today for your GMP, GLP or ISO 17025 needs. Please complete the form below to have an EAG expert contact you.