Surface Area and Pore Size Distribution via Gas Sorption Analysis
Home » Surface Area and Pore Size Distribution via Gas Absorption Analysis
A material’s surface morphology plays a vital role in its interactions with the surrounding
environment. Surface area and pore structure are important considerations in many fields,
impacting diverse industry concerns. Their distribution can be determined via Gas Sorption Analysis.
Excerpt
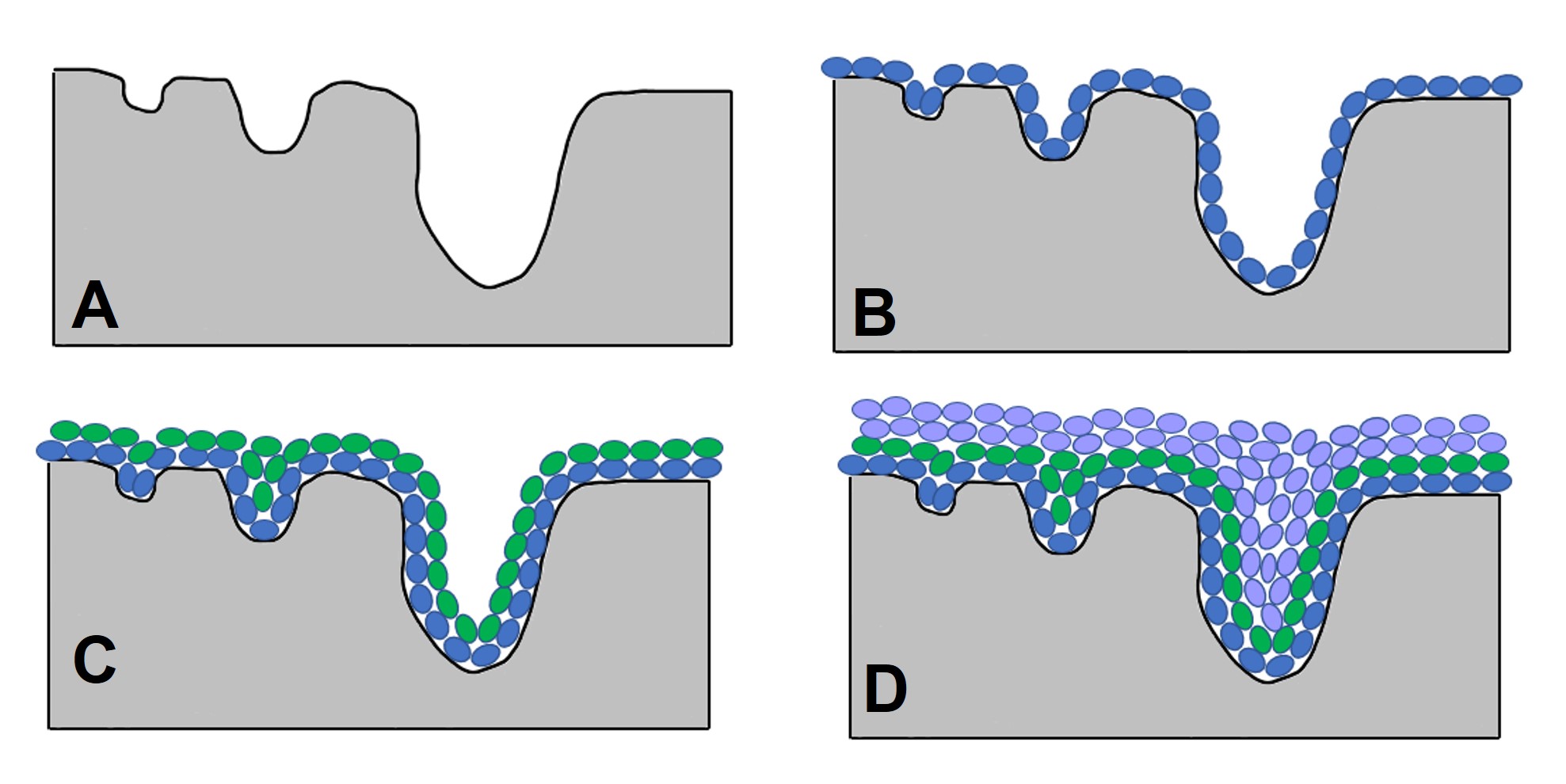
The measurement of a material’s surface morphology is commonly performed using nitrogen sorption analysis, where nitrogen molecules adsorbed on or desorbed from the sample surface, analyzed using the appropriate model, provide values for these parameters.
Gas sorption analysis takes advantage of the attraction between an open surface and any gas molecules it encounters. Interactions between the surface molecules of the sample (the adsorbent) and a gas molecule (adsorbate) lead to the gas molecules becoming weakly attached to the surface as liquids. A surface free of interactions (as
one is under vacuum) is in an unfavorable state, so when gas molecules are introduced to the adsorbent at low pressures, the molecules interact with the surface to form a film, as this is a more favorable state. Upon adsorption to the surface, these molecules are known as the adsorptive. This technique was originally developed to analyze mesoporous (pore diameter 2-50 nm) materials, though it can also be extended to microporous (< 2 nm diameter) and small macroporous (> 50 nm diameter) materials with the appropriate selection of analysis parameters.
Would you like to learn more about Gas Sorption Analysis?
Contact us today for your gas sorption analysis needs. Please complete the form below to have an EAG expert contact you.