Thermogravimetric Analysis (TGA–FTIR)
Home » Thermogravimetric Analysis (TGA–FTIR)
Commercial synthetic polymers such as epoxy resin, polyacrylonitrile, polyacrylates, phenolic resin, polyethyleneglycol, polyurethanes, polyolefins, polysiloxanes and fluoropolymers, are widely used in many industry sectors. These polymers feature backbones with -C-C-, -C-O-, -C-N-, -C-S-, Si-O- bonds, with substituents such as alkyl, aryl groups, -OH, -NH2, etc., which may be further modified with F, Cl, and Br. Under heat treatment either in air, under inert or reduced pressure conditions, they may release a variety of molecular species, including H2O, NH3, CO, CO2, HCN, HCNO, NOx, SO2, CSO, HF, HCl, HBr, CH4, C2H4, silanes, siloxanes, fluorocarbons, formaldehyde, phosgene, carbonyl fluoride and other volatile organics. These species not only lead to performance deterioration and ultimate failures, but also cause great safety and health concerns. As such, most of them are strictly regulated by EPA.
Mass spectrometric identification of these species generally requires vacuum conditions and identification quite often suffers from mass overlaps and poor ionization yields that makes detection and identification problematic. On the other hand, these molecular species have distinctive vibration patterns in the 450 cm-1 – 4000 cm-1 infrared regions (Figure 1). In fact, FTIR is the preferred method for analysis of combustion exhaust composition.
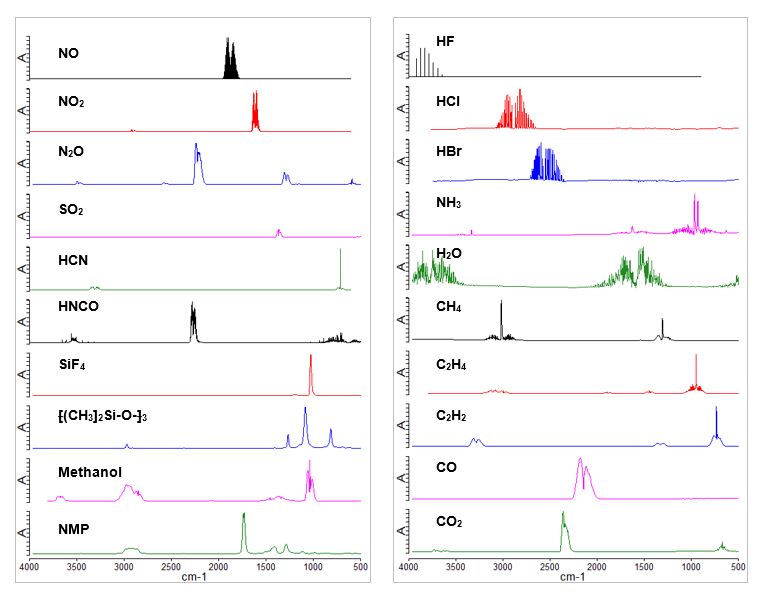
TGA-FTIR combines the strength of TGA with FTIR for materials characterization and outgassing profiling. It accurately records the mass loss as a sample is heated up in the TGA analyzer, and identifies the released molecular species resulting from the corresponding mass loss, by flowing the released molecular species through a long optical path gas cell of the FTIR instrument. At Eurofins EAG Syracuse Lab the above TGA-FTIR setup assures high sensitivity detection up to 10-100 parts per million mass loss for 50-100 mg sample.
CASE STUDY
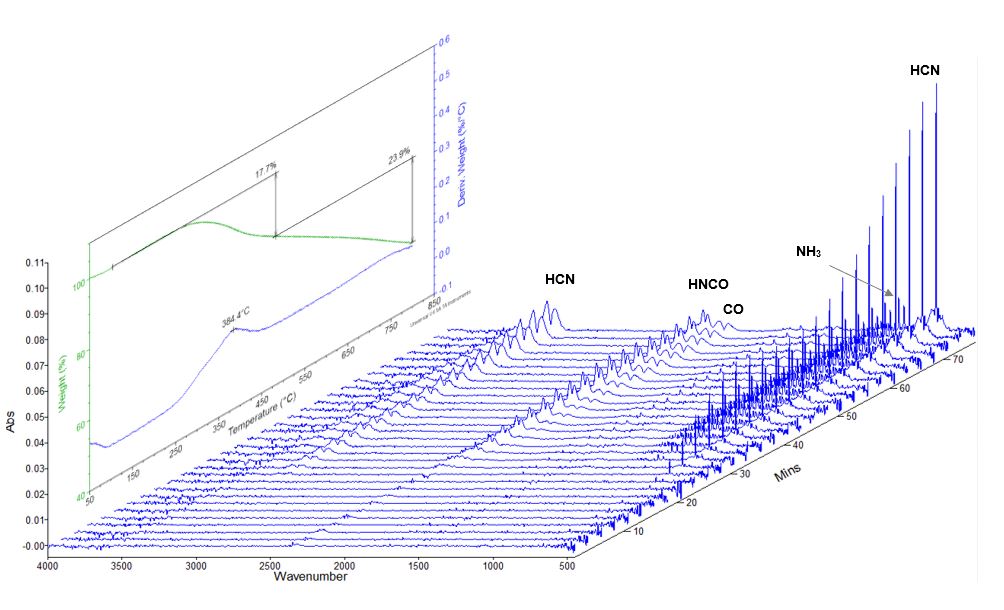
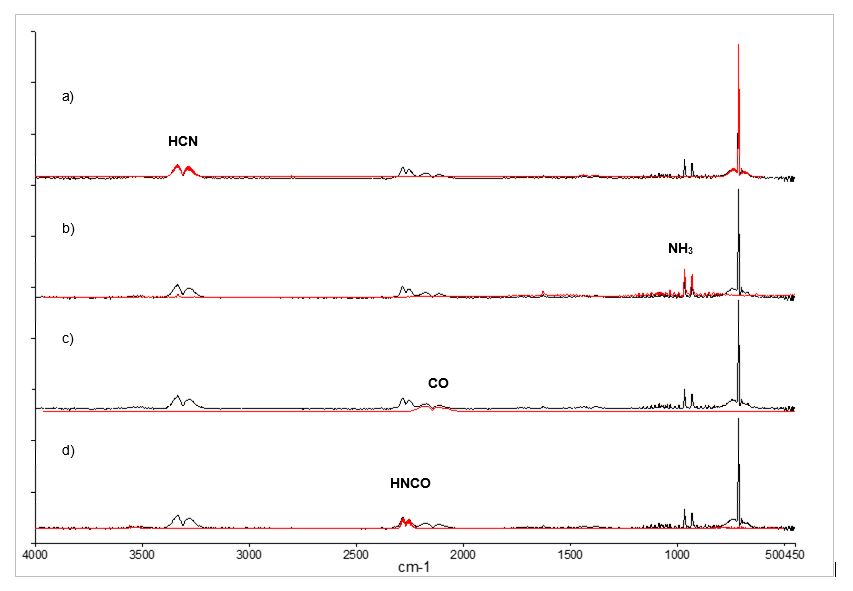
TGA-FTIR analysis is demonstrated with outgassing investigation of polyacrylonitrile (PAN). As carbon fiber precursor, PAN must go through a series of processes including thermo-oxidation stabilization, pyrolysis and graphitization, which are accompanied with intensive outgassing. TGA-FTIR provides a powerful insight to the pyrolysis kinetics and the outgassing profiles. Figure 2 depicts the time (temperature)-based outgassing profiles acquired on our TGA-FTIR instrument, for a thermo-oxidation stabilized PAN sample (Sigma-Aldrich), including CO, NH3, HCN, and HNCO, all highly hazardous air pollutants. The insert is the corresponding TGA profile, which shows the thermal decomposition pattern and pyrolysis kinetics. Figure 3 demonstrates the clear identification of the outgassing CO, NH3, HCN, and HNCO.
COMMON APPLICATIONS
- Survey analysis of hazardous outgassing
- Residual volatiles such as moisture, organic solvents, monomers
- Characterization of new or unknown materials
- Product deformulation
- Failure analysis
STRENGTHS
- Quantitative measurement of mass loss as a function of temperature and time
- Survey analysis of hazardous outgassing species such as H2O, NH3, CO, CO2, HCN, NOx, SO2, CSO, HF, HCl, HBr, CH4, silanes, siloxanes, fluorocarbons, phosgene, carbonyl fluoride, formaldehyde, and other organic volatiles
- Identying large molecular outgas species possible when combined with vacuum outgassing technique such as Direct Insertion Probe – Mass spectroscopy (DIP-MS)
- Allowing various working atmospheres, including inert atmosphere (argon and nitrogen), and reactive gas atmosphere such as air, H2/argon, and humidified argon;
- Programmable temperature control – flexible combination of dynamic heating and isothermal holding, from ambient to 1000 °C. In a special set up, the instrument can go up to 1500 °C.
LIMITATIONS
- High detection limits for low volatility outgassing species
- No detection of IR-inactive species such as H2, N2, O2, argon, etc
- Limited to ambient pressure outgassing testing only
TECHNIQUE COMPARISONS
Residual Gas Analysis (RGA). RGA is a high vacuum outgassing technique operating under 10-7 – 10-9 torr. This technique is suitable to quantitatively assess a whole range of small molecules (up to 100 amu), including H2, moisture, O2 and N2, etc. RGA is commonly used to assess internal water vapor content, which is known to affect the reliability of packaged electronic, medical, optical and devices. Testing methods such as MIL-STD-883, MIL-STD-750, Method 1018 Internal Water Vapor Content must be strictly followed to meet the DLA lab suitablity requirement.
Thermal Desorption Gas Chromotography Mass Spectrometry (GCMS) is used to analyze organic contaminants outgassed from solid sample matrices. GCMS separates and identifies individual organic species, up to 1000 amu. EAG’s thermal desorption chamber (1 1/2” diameter x 4” long) can be heated at any temperature between 45°C and 300°C, normally for a time period of one to three hours. Volatile organic components will be detected and semi-quantitative results will be provided. Detection limits are as low as 10 ng/component. Thermal Desorption GCMS cannot easily detect low level moisture, atmospheric species or other small molecules below 40 amu.
Would you like to learn more about Thermogravimetric Analysis (TGA–FTIR)?
Contact us today for your Thermogravimetric Analysis (TGA–FTIR) needs. Please complete the form below to have an EAG expert contact you.