Ultra-Trace Level Contaminants in materials using ETV–ICP–OES
Home » Ultra-Trace Level Contaminants in materials using ETV–ICP–OES
The advanced technologies have witnessed a sharply growing demand for developing and using powerful analytical methods with respect to high sensitivities, accuracy and analysis time for characterizing of trace and ultra-trace level of impurities on different specific properties of materials. Herein, we introduce electrothermal vaporization (ETV) coupled with inductively coupled plasma optical emission spectrometry (ICP-OES) as an exceptionally sensitive solid sampling technique for purity verifications of high-performance materials based on carbon, silicon, and silicon carbide.
These materials have wide technologically innovative applications and technical importance due to its outstanding properties useful for lithium-ion batteries, fuel cells, opto- and micro-electronics, water purification, fiber optics, spintronics, refractories, electric vehicles, etc [1-2]. Depending on materials purity, the future scope of a new market can also be identified. Thus, the trace and ultra-trace element contents and other chemical parameters are of eminent importance.
The traditional analytical techniques for purity evaluations of high-performance materials are very time-consuming and often tedious particularly for the decomposition of SiC by wet chemical procedures.
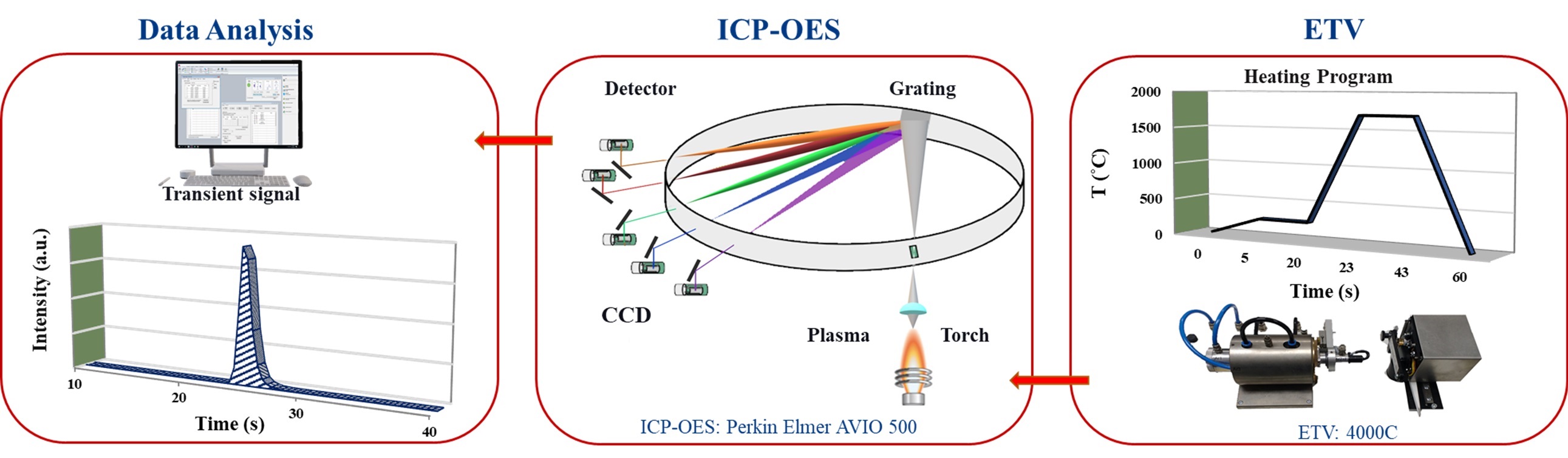
In this study, we demonstrated that ETV-ICP-OES is applicable for determining even low-volatility analytes especially the carbide-forming elements such as Cr, Ti, V, and Zr when halogenating reagents are used to form more volatile halides of the refractory elements. The whole ETV-ICP-OES process is presented in Figure 1. Various traceable calibration strategies typically certified reference materials (CRM) and aqueous standard solutions can be used for quantitative chemical analyses.
OPERATING PROCEDURE
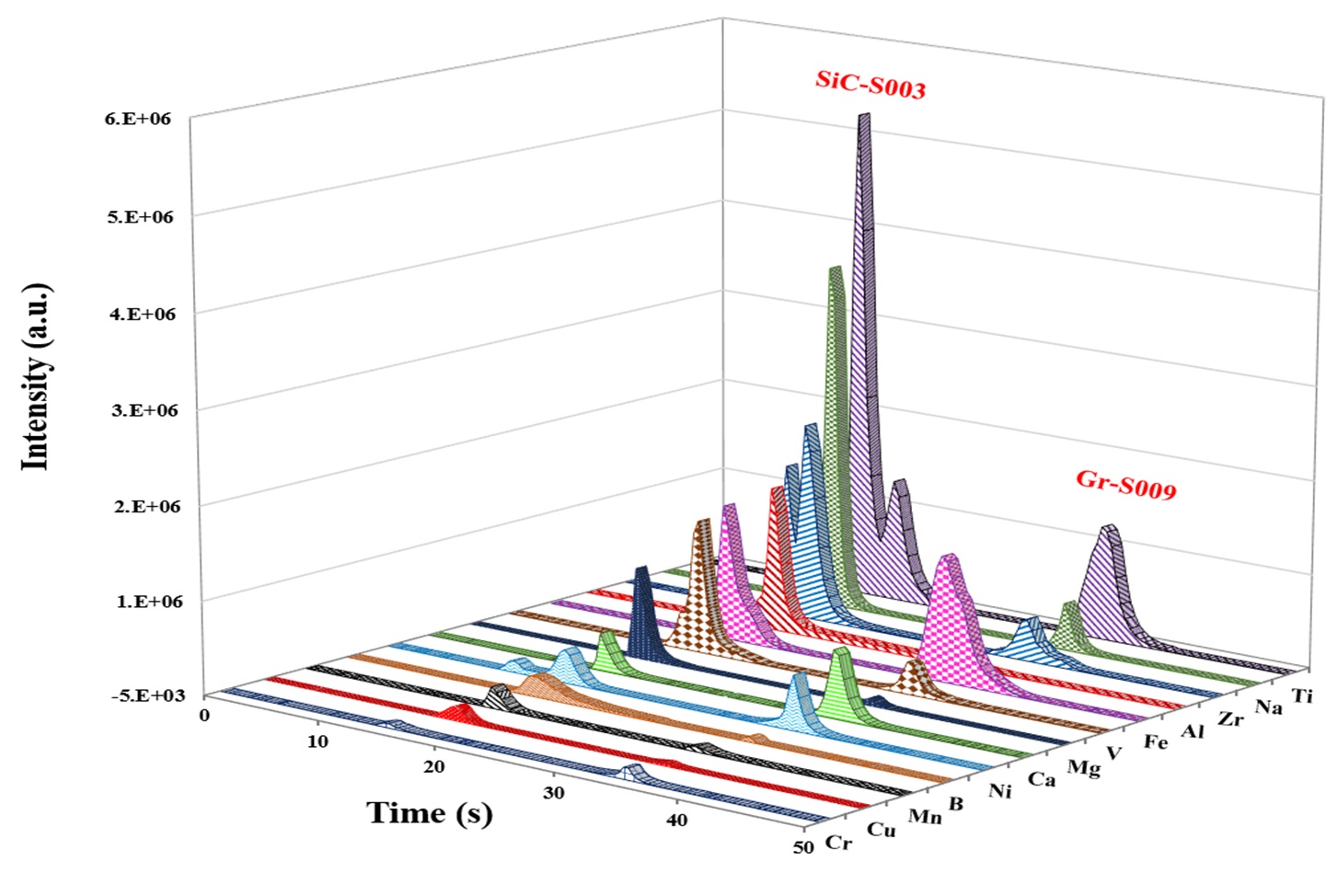
The employed ETV operational conditions were vaporization temperature of 1700 °C for all samples (Table 1). Various temperature programs can be used to separate elements in time which permits to minimize potential spectral interferences. Solid or liquid samples (<1 to 50 mg for solids; up to 50 μL for liquids) are placed in a pyrolytically coated, high purity graphite boat, that is resistively heated using an electronically programmable heating cycle. The boats are capable of attaining temperatures of up to 3000 °C in the ETV unit. Argon with 99.996% purity was used as protection, carrier, and bypass-gas on the ETV device. Analytes are vaporized in the presence of a halogenated modifier gas, UN1028 R12 (CCl2F2), and transported directly into the ICP (PerkinElmer, Avio 500) where they are excited, and detected by an optical emission spectrometer. Transient signals with changing intensities are generated in this method since ETV offers no constant sample introduction rate and the amount of sample introduced into the plasma varies with time. The typical transient signals of certain elements are displayed in Figure 2.
CALIBRATION
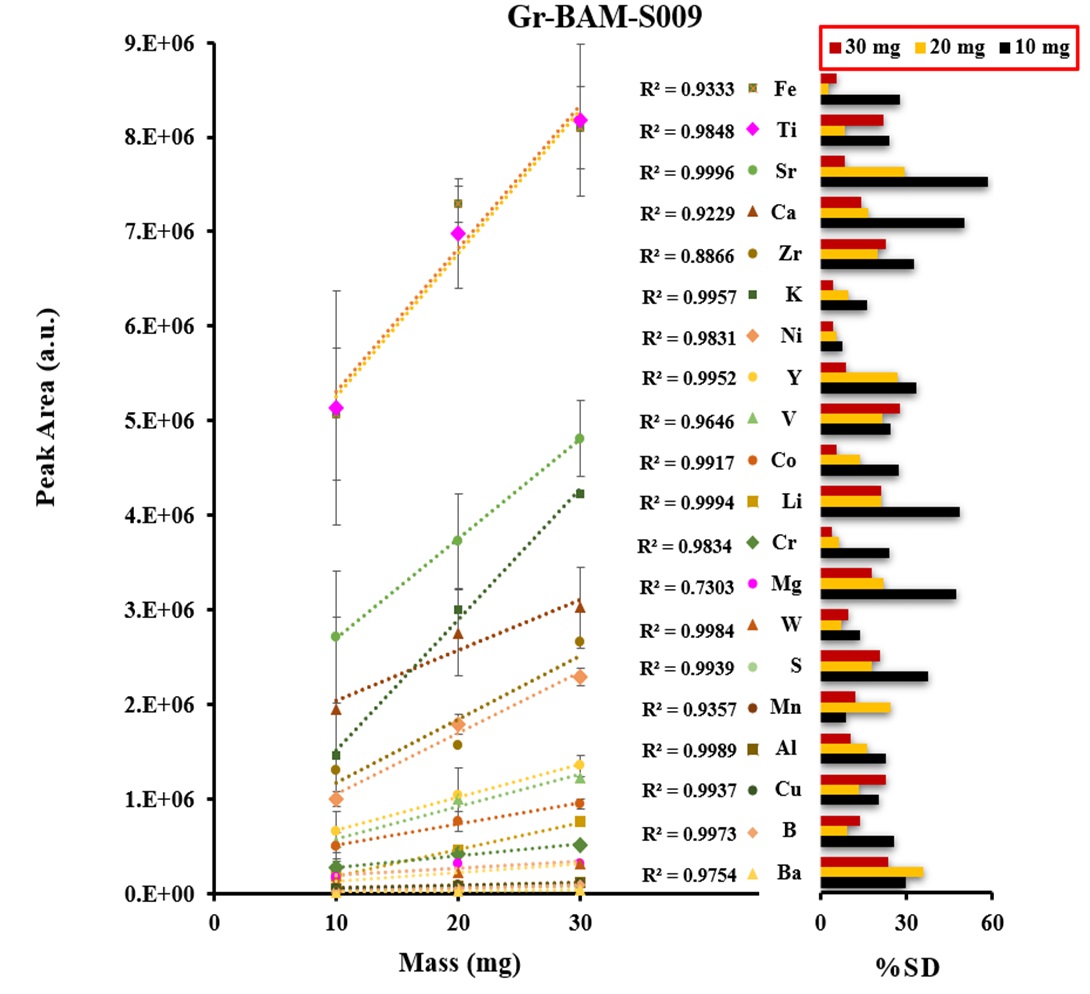
Certified reference materials, graphite (BAM-S009) and silicon carbide (BAM-S003), were used for the calibration. The calibration curves were obtained based on the three different masses of graphite and silicon carbide powders including a blank signal as shown in Figures 3 and 4. The calibration shows a linear trend and correlation coefficients were ranged from 0.951-0.999 for all analytes. The precision of emission signal measurements (peak area) was calculated for twenty and thirteen analytes of five replicates (n=5) in graphite and silicon carbide, respectively, which were ranged from 2.6% – 35.3 %RSD for the most of analytes.
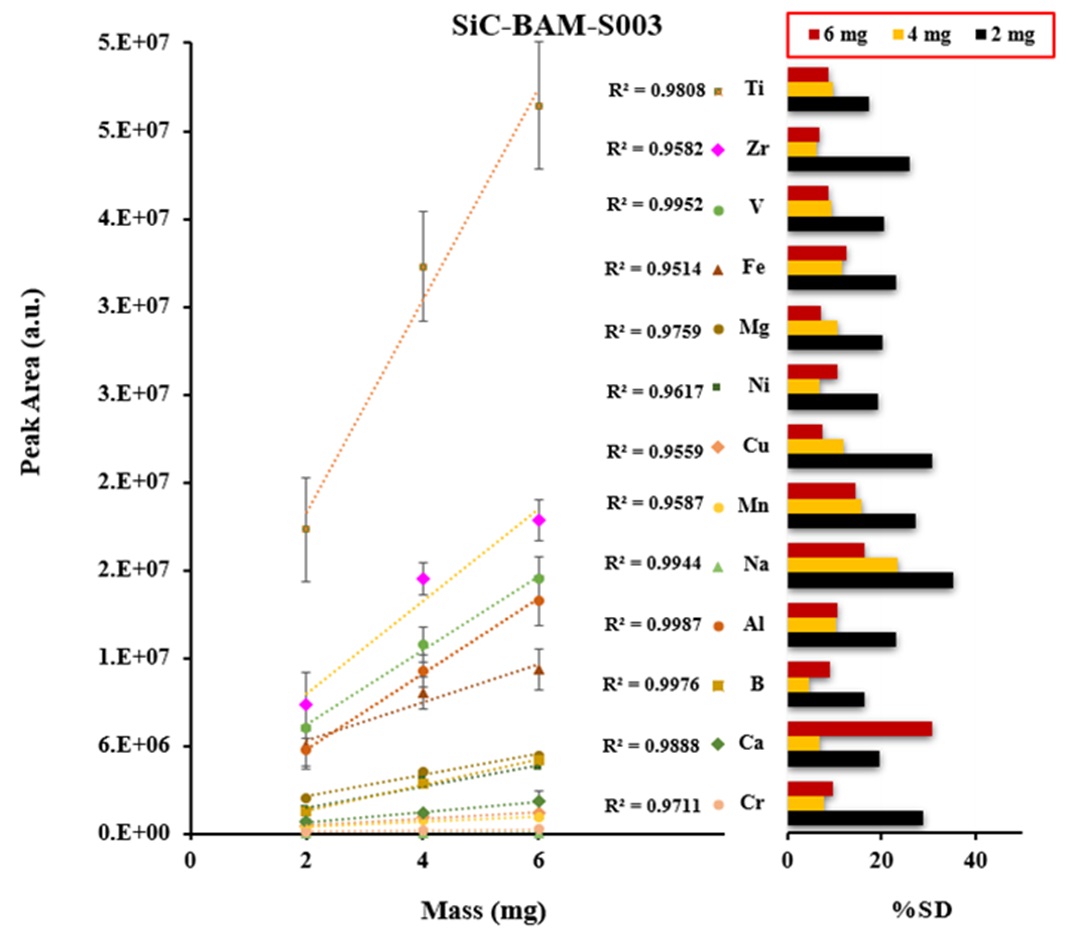
CASE STUDIES
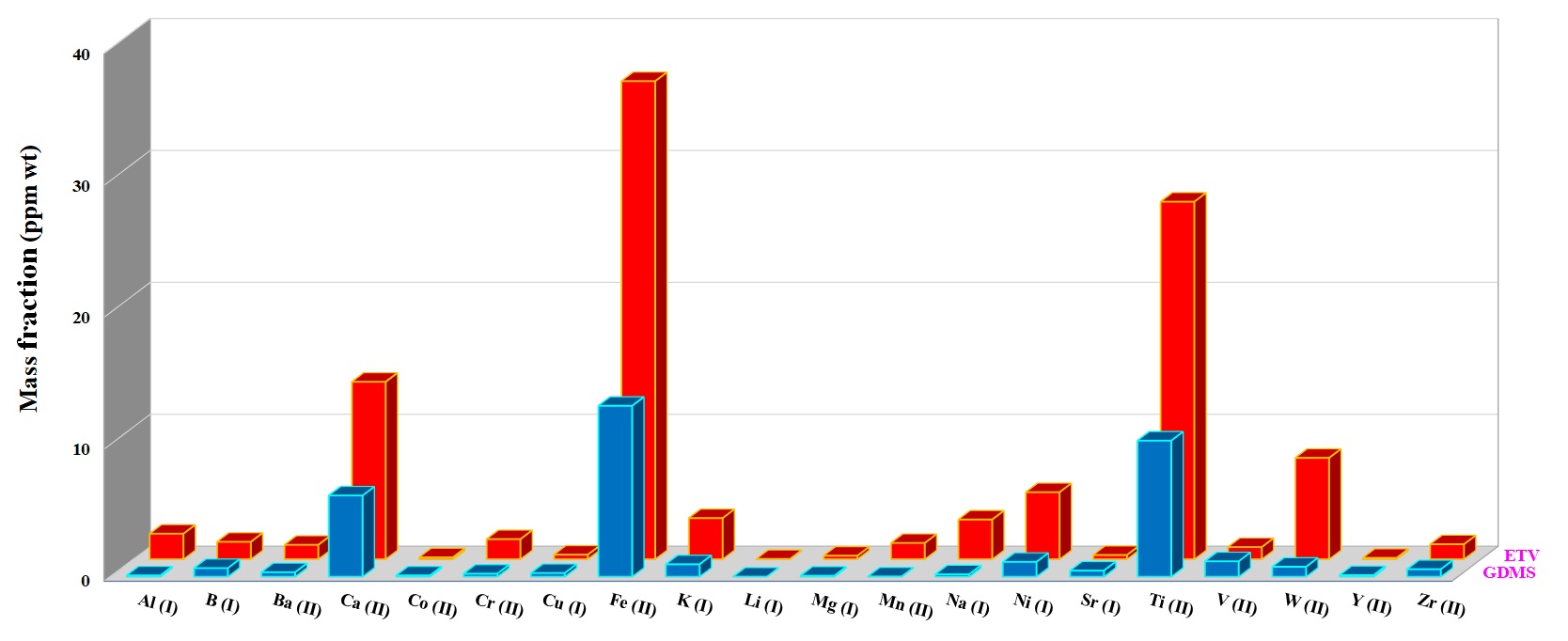
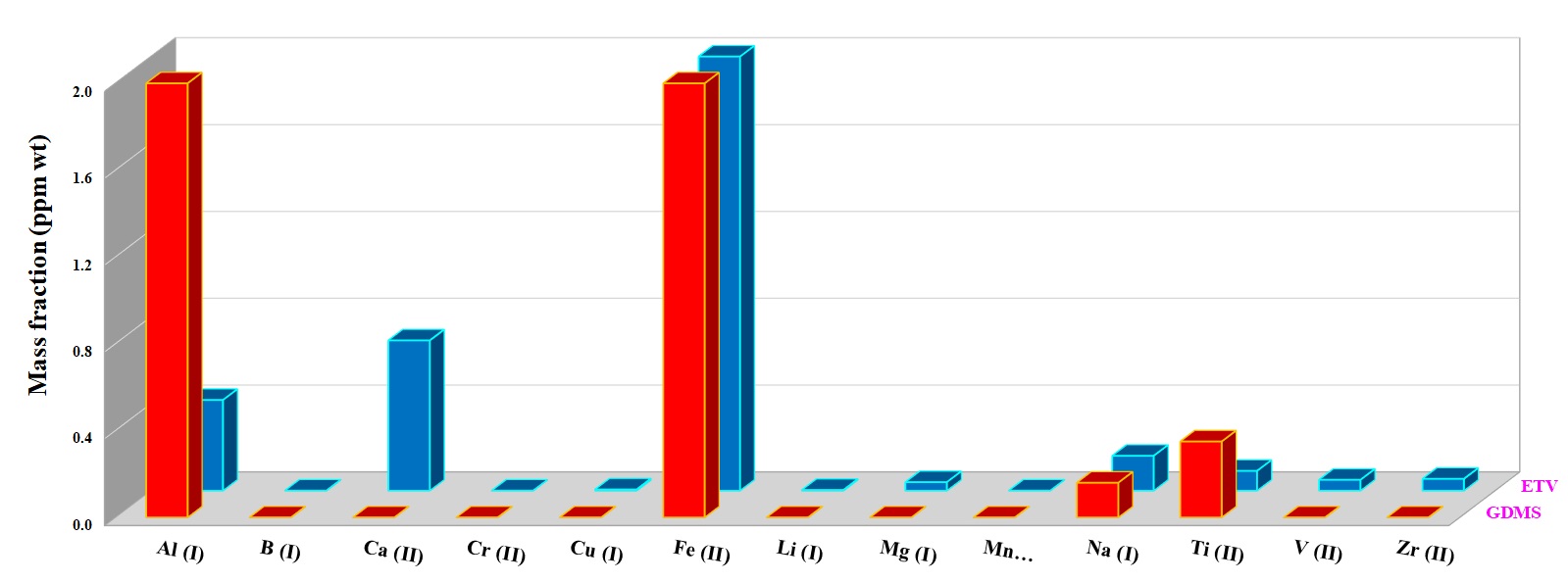
For quantification of the investigated elements in the nuclear-grade graphite and silicon carbide powder samples, ~5 mg of each sample was weighed directly into the graphite boat, transferred to ETV and then vaporized by applying the heating program given in Table 1 under R12 gas at 2.0 mL min−1. R12 as a reaction gas (CCl2F2) is among the most commonly used halogenated compounds. This gas decomposes at a temperature around 700 °C, producing CF2, CF3Cl, CF4, and C2F4 radicals, which promote the analyte conversion into volatile halides and finally increase the analyte transport as a result of cluster formation [3]. Figures 5 and 6 show the concentration of more volatile elements in the nuclear-grade graphite (NBG-18) and silicon carbide powders quantified by ETV-ICP-OES. The results by ETV-ICP-OES are compared to those obtained by Glow discharge mass spectrometry (GDMS) to validate the ETV-ICP-OES method. Derived results were found to be in good agreement with GDMS values, indicating the applicability of this new method for analysis of trace and ultra-trace constituents.
Would you like to learn more about ETV–ICP–OES?
Contact us today for your trace level contaminants in advanced material needs. Please complete the form below to have an EAG expert contact you.