XPS chemical shift and valence
Home » XPS chemical shift and valence
XPS (X-ray photoelectron spectroscopy) is capable of qualitative and quantitative analysis, and its most important unique feature is that it can also determine chemical states.
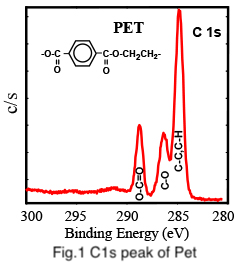
In the case of PET (polyethylene terephthalate), the carbon 1s peak consists of three peaks with different chemical shifts as shown in Fig. 1.: C-C / C-H, C-O, and O-C=O bonds can be seen in increasing order of binding energy.
The C-C / C-H bonds have the lowest binding energy for organic compounds. A decrease in electron density of the carbon atom relative to C-C / C-H bonds, increases the binding energy of carbon electrons. This chemical shift is dependent on the electronegativity (electron withdrawing power) of atoms bonded to carbon. Oxygen having more electron withdrawing power than carbon or hydrogen results in an increase in the C-O binding energy relative to C-C. Multiple bonds to electronegative atoms as in O-C=O increases the carbon binding even further.
Let’s look at the relationship between the oxidation state and the magnitude of chemical shift for typical metal oxides. As shown in Table 1, the amount of chemical shift increases as the oxidation state increases for each metal. The amount of chemical shift is also dependent on the electronegativity of the atoms surrounding the metal. For example the chemical shifts for metal oxides are smaller than the chemical shifts for metal fluorides. These trends are generally observed for most metals.
Element | Peak | Metal (0 valence) | Bivalent (2+) | Trivalent (3+) | Tetravalent (4+) |
|---|---|---|---|---|---|
| Ti | 2p3/2 | 454.1 | 455.0 (+0.9) | – | 459.0 (+4.9) |
| Fe | 2p3/2 | 707.0 | 709.3 (+2.3) | 710.9 (+3.9) | – |
| Ni | 2p3/2 | 852.7 | 853.8 (+1.1) | 856.5 (+3.8) | – |
Table 1 Binding energy of typical metals and their oxide peaks
(Units: eV The values in parenthesis are the energy differences from the metal peak)
As shown in Table 2 the binding energy is not always related solely to the electronegativity of the surrounding elements. In fact, the chemical shift for ionic solids is dependent on the overall electronic environment of the lattice (related to the Madelung energy of the solid) and to relaxation effects during the electron photoemission. Increasing the formal oxidation state of the atom and increasing the number of electronegative oxygen atoms surrounding the metal atom generally causes an increase in the binding energy but the increase is not a linear function of the either parameter.
| element | peak | Metal (0 valence) | Monovalent (1+) | Bivalent (2+) | Trivalent (3+) | Tetravalent (4+) |
|---|---|---|---|---|---|---|
| Co | 2p3/2 | 778.3 | – | 780.4 (+2.1) | 780.0 (+1.7) | – |
| Cu | 2p3/2 | 932.7 | 932.5 (-0.2) | 933.6 (+0.9) | – | – |
| Pb | 4f7/2 | 136.9 | – | 138.8 (+1.9) | – | 137.5 (+0.6) |
Table 2 Bond energy of typical metals and their oxides whose valence and chemical shift are reversed
Units: eV The values in parenthesis are the energy differences from the metal peak
The XPS binding energy of an atom is a measure of the electronic environment of the atom. Decreasing the electron density of an atom (such as a C-O bond compared with a C-C bond) increases the binding energy of the atom. Conversely increasing the electron density of an atom (such as fluoride ion F compared with fluorocarbon CF2) decreases the binding energy. As shown above for PET, binding energies for organic compounds are directly related to the hetero atom electronegativity and number of hetero atoms bonded to carbon.
For inorganic solids the binding energy is generally related to the formal oxidation state of atom. However secondary influences caused by the lattice structure and relaxation effects during the electron photoemission can results in chemical shifts that are at times smaller than might be expected. It is therefore necessary to compare measured binding energies with those obtained from pure compounds.
Would you like to learn more about XPS chemical shift and valence?
Contact us today for your XPS chemical shift and valence needs. Please complete the form below to have an EAG expert contact you.