Advanced Microscopy of Compound Semiconductors
In this webinar, we will explore the variety of microscopy techniques available at Eurofins EAG commonly utilized to characterize compound semiconductor materials.
Depending on our clients’ needs, labs within EAG have various certifications, accreditations, regulatory approvals and/or licenses.
For questions about our quality management system, you can reach our quality team here
EAG is committed to excellence in our professional practices, to offering world-class analytical testing services, and to continually improving our quality management system. Our mission to achieve total customer satisfaction by delivering on-time, accurate analyses is engraved in the backbone of our quality system. In keeping with this commitment, we have a number of controls in place to assure the effectiveness and efficiency of our quality system:
Our testing laboratories are registered with the DDTC (Directorate of Defense Trade Controls). A Technology Control Plan is in place to ensure compliance with all EAR (Export Administration Regulations) and ITAR (International Traffic in Arms Regulations). Our scientists and staff are experienced with the data security requirements in regards to EAR, ITAR and military standards.
You may view and download certifications and accreditations for many of our laboratories below.
International Quality Management Standard
International Standard for calibration and testing laboratories
International Quality Management Standard
For DPA and RGA testing our Labs are suitably equipped to perform testing on military devices for MIL-STD-750 and MIL-STD-883.
Covering electronic components, assemblies, related materials and processes

In this webinar, we will explore the variety of microscopy techniques available at Eurofins EAG commonly utilized to characterize compound semiconductor materials.

In this webinar we introduce the Testing of High Speed I/O to validate the devices from characterization to production

Different types of SIMS analyses exist. The two main types include Dynamic SIMS (high current) and static-SIMS (low current).
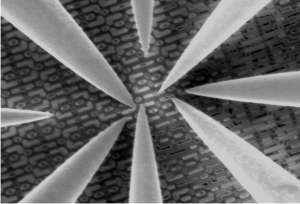
Nanoprobing is crucial for understanding advanced semiconductor devices, finding faults, and conducting thorough failure analysis.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.