Improving Performance and Safety of Lithium-Ion Batteries: Characterizing Materials and Interfaces
Home » Improving Performance and Safety of Lithium-Ion Batteries: Characterizing Materials and Interfaces
Lithium-ion batteries provide lightweight, efficient power for electric vehicles (EV) and consumer electronics. Although their future is bright because they offer higher energy density than other types of rechargeable batteries, two critical issues need to be addressed: performance and safety.
Performance is especially important for EV applications, where limited driving range and battery deterioration over time may reduce the attractiveness of EVs for consumers.
Safety is a serious concern. Reports of battery fires in smartphones as well as EVs have brought consumer safety issues to the forefront.
Systems or application engineers selecting batteries for use in end products understand voltage and footprint requirements, but may not be familiar with battery chemistry or failure mechanisms. This paper provides knowledge that may help them ask the right questions of potential suppliers, improving the chances that their product contains a battery optimized for their specific application.
This paper also serves as a starting point to learn more about lithium-ion batteries, focusing on information that will be helpful to engineers tasked with battery selection. Additionally, the information is valuable to battery designers, giving them insight that may help them collaborate more effectively with customers.
Lastly, this paper shows why proper battery characterization is important for improving performance and addressing safety concerns. We begin with an overview on how lithium-ion batteries work, discussing battery chemistry and trends in battery materials. We also explain what can go wrong. A wide variety of issues may contribute to battery degradation or failure, and understanding the cause of failure is complex.
We describe various in-situ and destructive characterization techniques, with examples of failure analysis applications. This information can help engineers select an appropriate battery characterization technique as well as know what questions to ask when working with an independent testing facility. We cover techniques for both imaging and chemical analysis.
Proper battery disassembly procedures are critical when performing destructive analysis. We include an overview of issues to be aware of, emphasizing the need for properly trained personnel.
INTRODUCTION: GROWING DEMAND FOR LITHIUM-ION BATTERIES
Lithium-ion batteries are lightweight and provide higher energy density than lead-acid or nickel–metal hydride (NiMH) batteries, creating a demand for them in electric vehicles (EV), energy storage, and consumer electronics. Compared to NiMH batteries, lithium-ion batteries have a 50 percent greater capacity in watt-hours per kilogram (w-h/kg).
The EV industry is demanding higher-efficiency batteries; in response, vehicle manufacturers are stepping up lithium-ion battery production. For example:
- Tesla is building a “gigafactory” in Nevada that it claims will produce enough lithium-ion batteries to support its planned production rate of 500,000 cars per year by 2020.1
- All commercial EVs contain lithium-ion batteries, as do plug-in hybrid vehicles. With 18 manufacturers currently selling electric or plug-in models, and more models to come in 20172, this is an encouraging market for battery manufacturers.
- Toyota is offering lithium-ion batteries in its higher-end Prius hybrid vehicles, allowing those cars to achieve the same mileage as more-stripped-down models with NiMH batteries, even though they contain options that add additional weight.3
Critical Issue: Improving Performance
Despite the advantages of lithium-ion batteries, they suffer from some challenges that may delay widespread adoption. Operating life and total life span remain the primary issues.
Operating life between charges is greater in today’s batteries than it was in older designs, but it still leaves ample room for improvement, especially for EVs. Mobile phone batteries can last an entire day before they need charging, even when running demanding applications, which is sufficient for most consumers. When they need charging, it is easy to find an outlet and charge the phone within an hour. EV range, while improving, is limited to 100 miles or less on many vehicles. Charging stations are not always conveniently located. Standard level 2 chargers take many hours to charge a depleted battery, making them impractical for drivers needing to travel long distances.
Lithium-ion battery performance degrades over time, at a rate that depends on battery materials and design as well as end use. Battery performance can deteriorate for multiple reasons, as described in the “Common Battery Failures” section of this paper.
Lithium-ion batteries used in mobile phones and laptops last only a few years, after which they can no longer hold a charge. Those used in EVs must be much more rugged, and many come with 8- to 10-year, 100,000-mile warranties. Still, charging capacity will likely decrease over that period, decreasing the resale value of the car.
Critical Issue: Addressing Safety Concerns
The recall of Samsung Galaxy Note 7 phones in September 2016 again brought lithium-ion battery safety concerns to the forefront. Samsung initially believed the culprit was a manufacturing defect from a specific third-party battery supplier that caused the devices to catch fire. The company offered customers free replacement batteries from a different supplier. When phones with the replacement batteries also caught fire, Samsung responded by ceasing manufacture of all Galaxy Note 7 phones in October. As of January 2017, nearly all the 3 million
Note 7 phones sold have been returned to Samsung. The Samsung story underscores the importance of designing batteries with the end application in mind and conducting thorough system-level tests. While Samsung continues to blame battery design, it is likely that the power and footprint demands of that specific phone played a contributing role.
Several dramatic incidents involving Tesla cars, during which the cars were totally consumed within minutes after a battery fire started, drive home the critical importance of lithium-ion battery reliability. Tesla made a design change to its Model S vehicles in 2014 to address this issue, adding titanium and aluminum underbody shielding so that road debris cannot easily penetrate the battery pack.4 Despite this measure, two Model S vehicles caught fire in 2016.5,6
Lithium-ion batteries are more prone to catching fire than other types of batteries. Even though the number of battery fires is extremely small compared with the number of batteries in service, the risk of fire must be drastically reduced to give consumers confidence that lithium-ion batteries are safe. The prospect of an EV catching fire is frightening.
Fires occur when shorts between the positive and negative electrodes cause the battery to heat up to an unsafe temperature. To understand why this is a greater risk with lithium-ion batteries and how to minimize this risk, users need to understand how lithium-ion batteries work and what can go wrong.
UNDERSTANDING LITHIUM-ION BATTERIES
How Do They Work? Battery Chemistry
Lithium-ion batteries work in a similar manner to all rechargeable batteries, in which reversible chemical reactions inside each cell cause ions to move between the cathode (positive electrode) and the anode (negative electrode). When the battery is being charged, an external electric current is applied, and lithium ions move from the cathode to the anode. During discharge, the lithium ions migrate back to the cathode and release energy, which powers the device. The cell is filled with an electrolyte through which the ions travel.
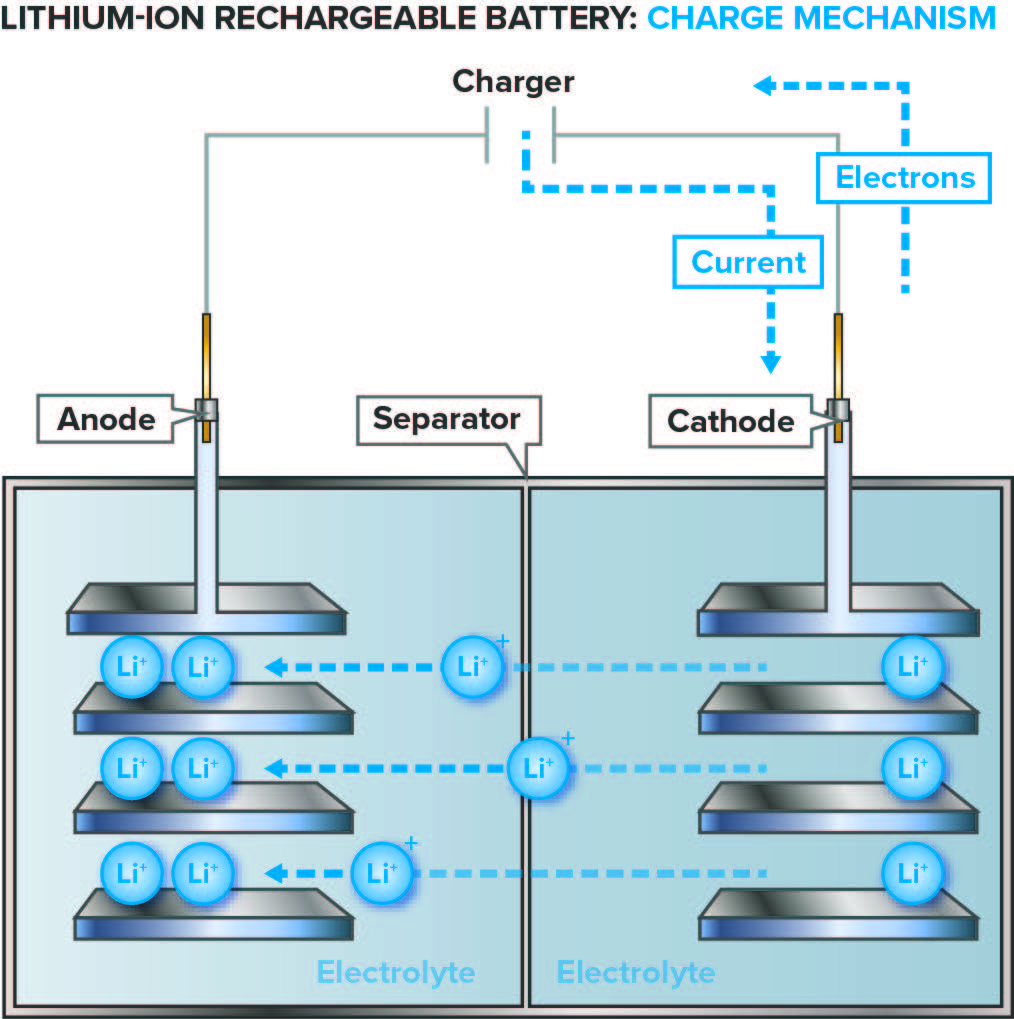
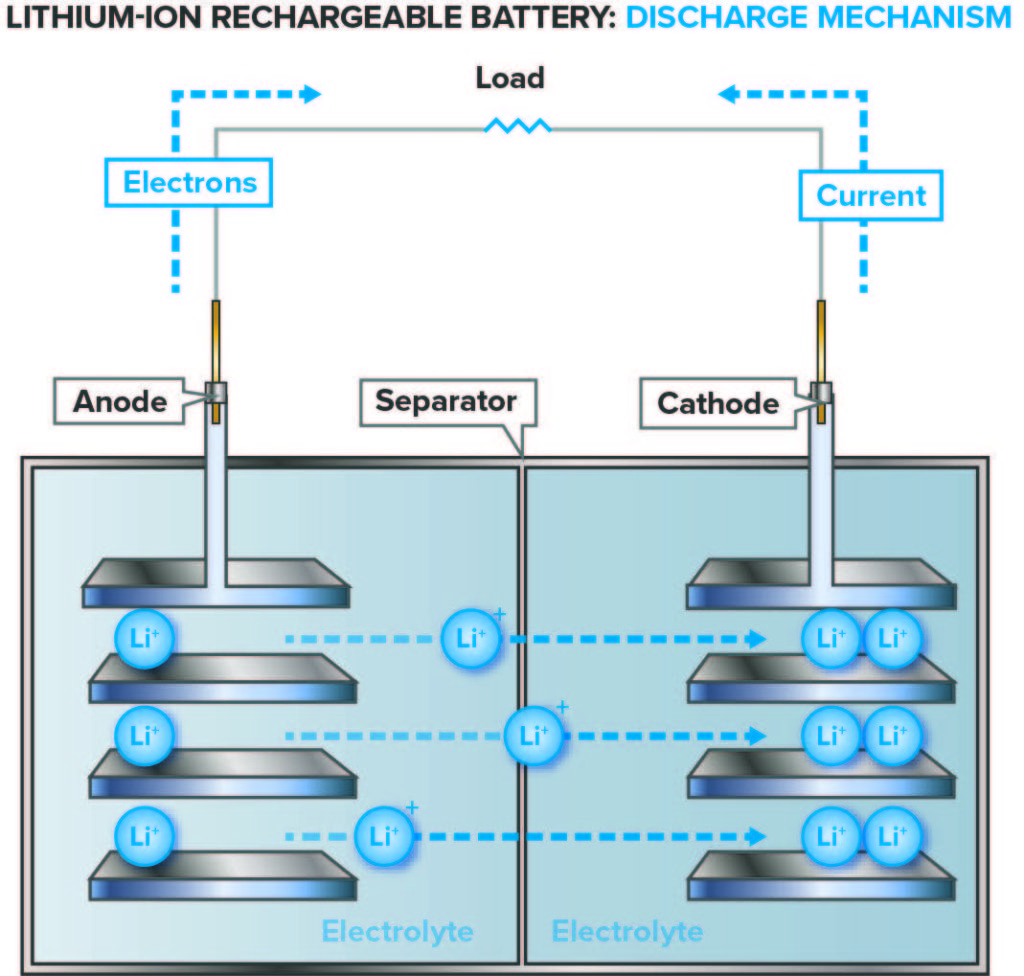
Figures 1a and b: Diagram of the process; Lithium-ion rechargeable battery charge and discharge mechanism.
(Source: Image courtesy of Marshall Brain, originally appearing in “How Lithium-ion Batteries Work,” November 14, 2006. HowStuffWorks.com, http://electronics.howstuffworks.com/everyday-tech/lithium-ion-battery.htm)
Each battery cell contains a separator, usually a polymer membrane, to electrically isolate the anode from the cathode. The integrity of the separator is critical to battery safety.
Lithium is a very reactive metal; this is a valuable characteristic for energy storage, but it also makes lithium-ion batteries potentially risky. If the internal battery temperature becomes too high, reactions will speed up to the point of being unstable.
Battery design can incorporate features that minimize the chance of this thermal runaway, such as adding safety vents to release internal pressure and using microporous separators that fuse above a certain temperature, blocking excess ion transport.
As batteries age, the chemicals inside the cells become depleted with each charging cycle, resulting in reduced capacity over time. Solid-electrolyte interphase (SEI) layers build up on the electrodes, limiting further ion transport.
Each battery cell contains a separator, usually a polymer membrane, to electrically isolate the anode from the cathode. The integrity of the separator is critical to battery safety.
Lithium is a very reactive metal; this is a valuable characteristic for energy storage, but it also makes lithium-ion batteries potentially risky. If the internal battery temperature becomes too high, reactions will speed up to the point of being unstable.
Battery design can incorporate features that minimize the chance of this thermal runaway, such as adding safety vents to release internal pressure and using microporous separators that fuse above a certain temperature, blocking excess ion transport.
As batteries age, the chemicals inside the cells become depleted with each charging cycle, resulting in reduced capacity over time. Solid-electrolyte interphase (SEI) layers build up on the electrodes, limiting further ion transport.
Trends in Battery Materials
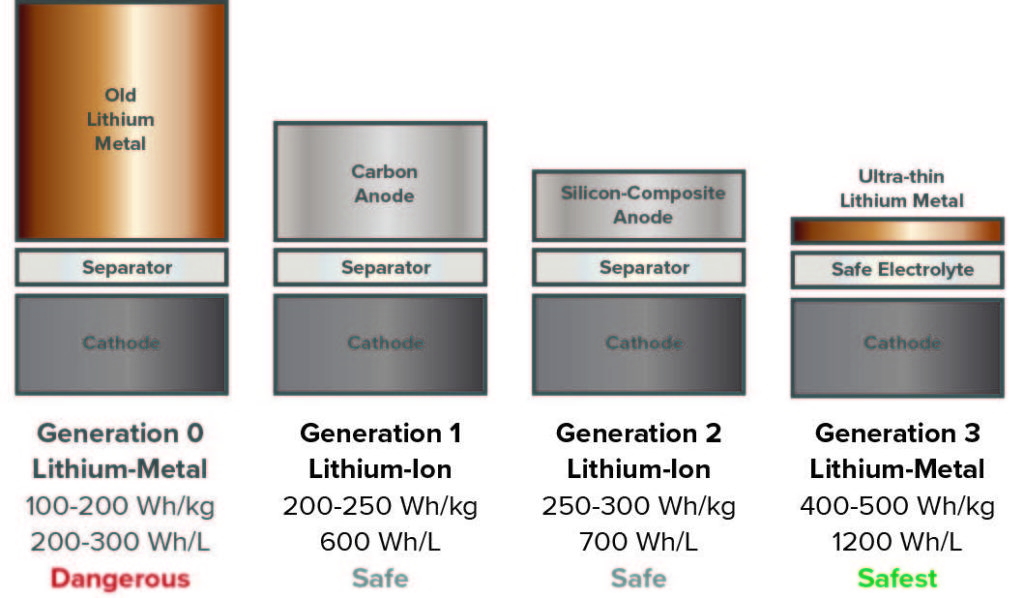
Lithium-ion batteries have seen improvements in materials and assembly processes since Sony commercialized the technology in 1991.7 U.S. patents issued in the 1990s describe advances in foil morphology and electrolyte materials that are now common in lithium-ion batteries.8,9
Cathode Materials. The original cathode material is lithium cobalt oxide (LiCoO2). Some commercial lithium-ion batteries contain lithium iron phosphate (LiFePO4) or lithium manganese oxide (LiMn2O4) cathodes.
Combining lithium manganese and lithium nickel manganese cobalt oxide (NMC) cathode materials produces a battery with an optimum combination of acceleration and driving range for EVs. Vehicles such as the Nissan Leaf, Chevy Volt, and BMW i3 run on NMC batteries. Tesla vehicles use a lithium nickel cobalt aluminum oxide (NCA) battery.
Anode Materials. Most lithium-ion batteries contain a carbon anode, in the form of graphite. Lithium metal anodes are attractive because of their high energy density, but they are prone to forming dendrites that can cause shorts, increasing the risk of battery fires. Silicon-based anodes are another option to increase energy density, but formation of SEI layers can decrease reliability.
MIT spinoff SolidEnergy Systems is commercializing a battery with a thin lithium foil anode, which is said to double the energy capacity compared to a standard lithium-ion battery. The company has created a new electrolyte designed to minimize the risk of shorts.
Electrolytes. The electrolyte can be liquid or solid (gel) and is usually polymer-based, consisting of organic solvents and lithium salts. Liquid electrolytes more efficiently transfer lithium ions, but they are highly flammable; solid electrolytes are less conductive, but they are safer because they are not likely to catch fire. Materials selection can improve the safety of liquid electrolytes. One example is lithium hexafluorophosphate (LiPF6) dissolved in a mixture of ethylene carbonate and diethyl carbonate. The mixture of solvents is more stable than either solvent used by itself.
Battery Form Factors for Various Applications
Each battery cell contains layered sheets of anode and cathode materials enclosed in a metal case. A copper foil is coated with anode material (typically graphite powder), and an aluminum foil is coated with cathode material; these are sandwiched with a polymer separator and either stacked vertically or, more commonly, wound into a coil.
Once the electrodes are enclosed in an aluminum case or foil pouch, the case is filled with electrolyte before sealing. Most batteries include a release valve to exhaust gaseous byproducts that form as the electrolyte breaks down during use. The cell shape may be cylindrical, similar to a standard alkaline AA battery, or prismatic (square or rectangular).
A battery pack consists of multiple interconnected cells. Linking cells in series increases the voltage at which the battery operates, and combining multiple cells or rows of cells in parallel increases the current that the batteries can withstand.
Common Battery Failures
Lithium-ion batteries may fail catastrophically when shorts occur between the anode and the cathode. A number of manufacturing defects inside the cells may increase the likelihood of such failure. These include:
- Burrs on the electrode foils
- Voids in the electrode materials
- Chemical contamination
- Inconsistent particle morphology of electrodes
Applications engineers sourcing lithium-ion batteries have no way to easily identify these defects. Accelerated life testing during qualification helps ensure batteries will perform safely and according to expectations.
Batteries can also be considered failed when capacity has decreased beyond a certain point. Overheating and operating at high voltage can accelerate the mechanisms that reduce battery life. Several different problems may occur during use:
- Loss of electroactive ions, leading to decreased power density
- Excessive growth of passivation layers on the electrodes
- Breakdown of the solvent or lithium salts in the electrolyte
- Swelling of electrodes, which irreversibly increases the discharge rate
- Pressure buildup, which can rupture sealed cells
- Delamination of electrodes from metal foils
- Cracking of electrodes due to mechanical stresses
The chart below illustrates the various mechanisms that can cause degradation of lithium-ion battery cells.
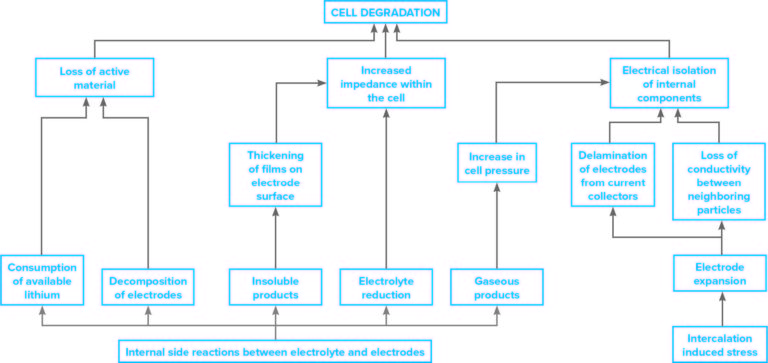
CHARACTERIZING LITHIUM-ION BATTERIES
The Need for Characterization and Failure Analysis
As battery manufacturers and end users demand higher performance and increased safety, characterization becomes increasingly important to identify manufacturing and design defects.
Batteries need to be evaluated using both in-situ and destructive testing to determine how to best improve performance and safety, using materials and processes that will be effective without increasing battery cost.
The selection of an appropriate characterization technique depends on what information is needed, at what level of accuracy, and the budget for qualification and testing. Nondestructive testing has the benefit of avoiding the need to disassemble the battery, but the level of information that can be extracted is limited. The most precise techniques tend to use the most expensive instruments and take the most time, but they are sometimes needed in order to understand failure mechanisms and improve battery design.
Optical, X-ray, and Electron Microscopy: High-resolution Imaging of Layers and Interfaces
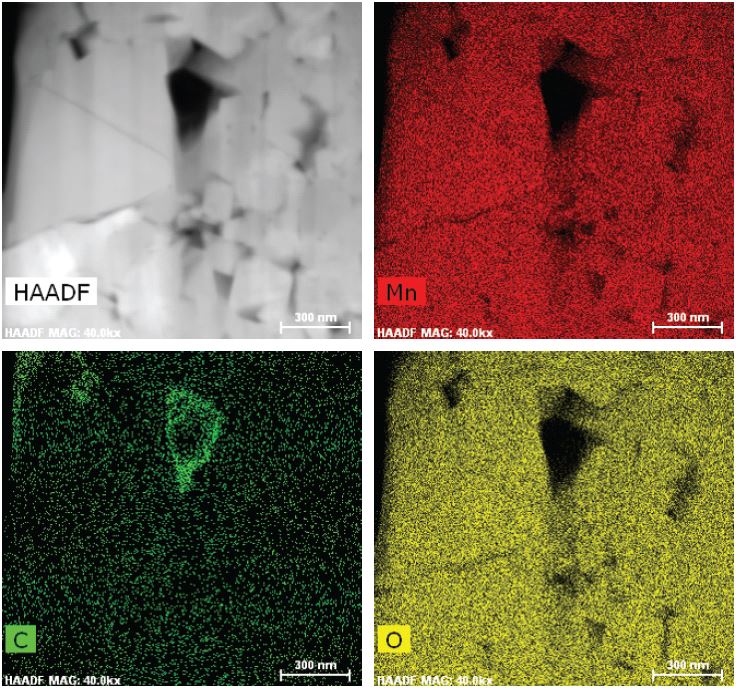
Microscopy techniques are used to image the various layers in a battery. Optical microscopy may be sufficient for examining larger cracks, but either scanning electron microscopy (SEM) or transmission electron microscopy (TEM) is required to measure layer thickness and observe changes in the microstructure, such as microscopic holes (voids) and defects.
SEM and TEM are inherently destructive techniques, but ion milling can preserve the integrity of the sample so that it accurately represents the original state of the battery materials before testing.
Researchers at Carl Zeiss and University College London used optical and X-ray microscopy, as well as SEM, to examine the microstructure of a commercial lithium-ion battery at length scales ranging from 20 µm down to 100 nm (0.1 µm).10 The researchers also examined batteries before and after cycling, imaging cracks that formed on the outer side of the roll and demonstrating the importance of imaging-characterization techniques to identify microstructure-based performance degradation.
The SEI layer caused by electrode-electrolyte interfacial reactions is so thin that it can only be visualized by TEM. Researchers at Oak Ridge National Laboratory used this technique, for example, to analyze dendrites that grow from defects in the SEI and form in lithium anodes.11 TEM combined with X-ray diffraction can be used to analyze the phase transformations associated with the diffusion of lithium ions through the electrode.
The Need for Characterization and Failure Analysis
As battery manufacturers and end users demand higher performance and increased safety, characterization becomes increasingly important to identify manufacturing and design defects.
Batteries need to be evaluated using both in-situ and destructive testing to determine how to best improve performance and safety, using materials and processes that will be effective without increasing battery cost.
The selection of an appropriate characterization technique depends on what information is needed, at what level of accuracy, and the budget for qualification and testing. Nondestructive testing has the benefit of avoiding the need to disassemble the battery, but the level of information that can be extracted is limited. The most precise techniques tend to use the most expensive instruments and take the most time, but they are sometimes needed in order to understand failure mechanisms and improve battery design.
Optical, X-ray, and Electron Microscopy: High-resolution Imaging of Layers and Interfaces
Microscopy techniques are used to image the various layers in a battery. Optical microscopy may be sufficient for examining larger cracks, but either scanning electron microscopy (SEM) or transmission electron microscopy (TEM) is required to measure layer thickness and observe changes in the microstructure, such as microscopic holes (voids) and defects.
SEM and TEM are inherently destructive techniques, but ion milling can preserve the integrity of the sample so that it accurately represents the original state of the battery materials before testing.
Researchers at Carl Zeiss and University College London used optical and X-ray microscopy, as well as SEM, to examine the microstructure of a commercial lithium-ion battery at length scales ranging from 20 µm down to 100 nm (0.1 µm).10
The researchers also examined batteries before and after cycling, imaging cracks that formed on the outer side of the roll and demonstrating the importance of imaging-characterization techniques to identify microstructure-based performance degradation.
The SEI layer caused by electrode-electrolyte interfacial reactions is so thin that it can only be visualized by TEM. Researchers at Oak Ridge National Laboratory used this technique, for example, to analyze dendrites that grow from defects in the SEI and form in lithium anodes.11 TEM combined with X-ray diffraction can be used to analyze the phase transformations associated with the diffusion of lithium ions through the electrode.
XPS: Measuring Electrode and Electrolyte Composition and Chemistry
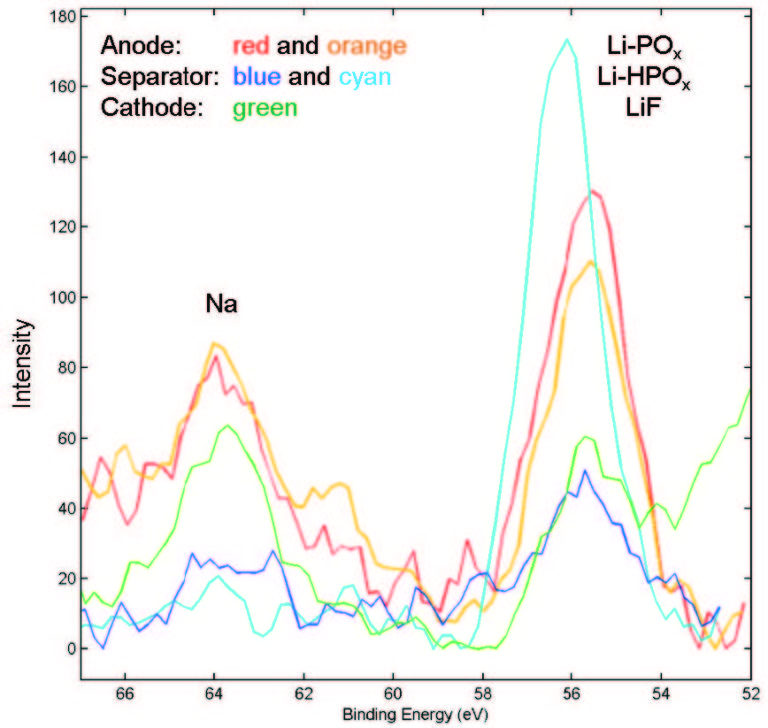
X-ray photoelectron spectroscopy (XPS) is a valuable tool for analyzing the structure and composition of the various layers in a battery, showing migration of lithium and other elements within the cathode, anode, and separator. XPS provides detailed quantitative information that can aid in failure analysis or that can help show how changes in materials or design affect SEI formation.
XPS has been used along with time-of-flight secondary ion mass spectrometry (TOF-SIMS) to analyze SEI formation in silicon-based anodes, looking at both structure and composition to understand how additives can improve stability of the SEI layer.12 The Vehicle Technologies Office, U.S. Department of Energy under the Advanced Battery Materials Research Program funds this research with the aim of improving batteries for EVs.
GDMS: Monitoring Impurities
Glow discharge mass spectrometry (GDMS) is useful for detecting trace quantities of elements. In battery applications, this technique can be used to identify impurities and contaminants that may adversely affect battery performance.
One precaution with this technique is that improper battery disassembly can introduce contaminants that were not already present in the battery.
FTIR: Studying Chemical Processes During Battery Operation
Fourier transform infrared (FTIR) spectroscopy is another method for analyzing chemical composition. This technique has been used to analyze components of the SEI layer that forms as an electrolyte decomposes with battery cycling as a function of applied voltage.13
ICP-OES: Tracking Performance of Cathode
Inductively coupled plasma optical emission spectrometry (ICP-OES) is used to detect the presence of trace metals by heating and ionizing the species in a sample. EAG Laboratories developed a technique to separate the cathode from a battery cell in various charging states or after a certain number of charge/discharge cycles. The researchers then used ICP-OES to precisely measure levels of lithium and manganese in the cathode, measuring small changes in lithium content that correlate with decreased battery performance.
GCMS: Investigating Swollen Batteries
Gas chromatography–mass spectrometry (GCMS) for battery applications requires siphoning gases from a hole drilled into a battery cell to analyze the gases released during breakdown of the electrolyte. This technique is especially helpful in cases where the battery has swollen or experienced thermal runaway, as these failure mechanisms are associated with premature electrolyte breakdown.
Raman Spectroscopy: Phase Analysis
Raman spectroscopy measures the vibrational energy in molecules in order to identify chemical species. By combining the technique with optical microscopy, confocal Raman microscopy provides details on the spatial distribution of phases within a sample. It is possible to determine, for example, whether charged lithium particles are distributed uniformly or grouped in clusters on a cathode.
Raman microscopy is useful for understanding SEI formation in lithium-ion batteries, either for failure analysis or for research into improving battery materials. The U.S. Army Research Laboratory and the Center for Research in Extreme Batteries, for example, used confocal Raman microscopy to analyze interphases that develop at the interface between the electrolyte and the cathode in lithium-ion batteries as part of their research on developing advanced electrolytes.14
THE IMPORTANCE OF INDEPENDENT TESTING
Battery manufacturers test and qualify batteries before shipping them to customers, but it still makes sense for customers to do their own qualification to ensure the batteries are safe and that they perform to the claimed specifications. This is good business practice, especially when switching vendors or products, but also as a matter of course for proper quality control.
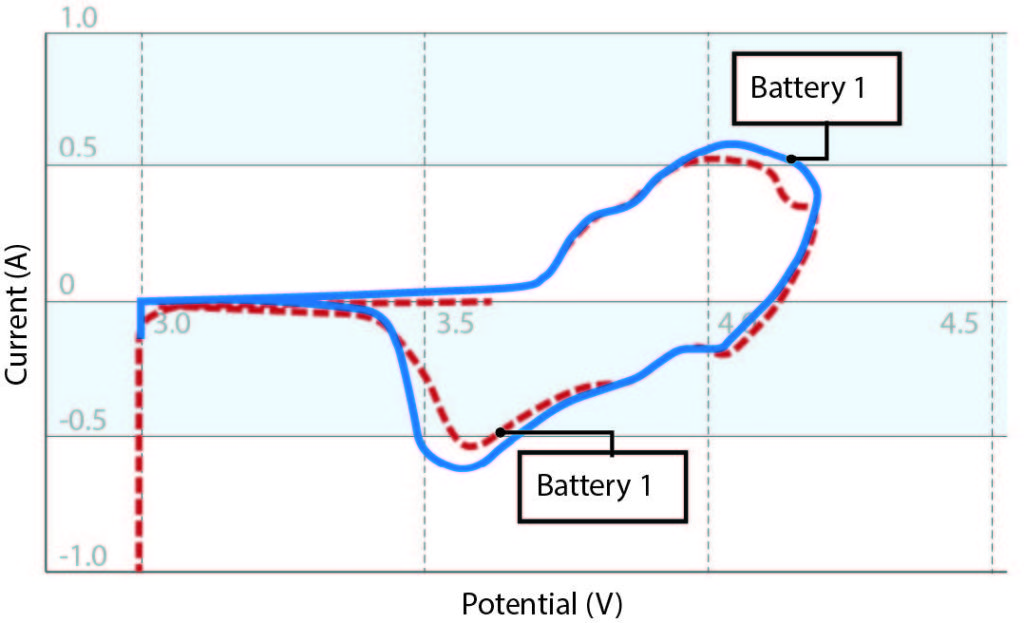
It is also important to conduct failure analysis on defective batteries to understand the source of the problem. Cyclic voltammograms (see Figure 2) measure drops in discharge current over time, but they do not explain why battery capacity has decreased. Thorough failure analysis will most likely require disassembling the battery, a process that must be done properly to ensure operator safety and maintain the operational state of the battery.
Battery Disassembly Procedures
While various standards address manufacture, testing, and transport of lithium-ion batteries, no existing standards address lithium-ion battery disassembly. Researchers at the University of Maryland have developed a procedure for best practices.15
Disassembly needs to be conducted in a controlled environment, typically an argon-filled glove box, to avoid introducing moisture or contaminants and to protect the technician from inhaling toxic compounds. It is important to consult battery manufacturers’ materials safety data sheets to understand what materials the battery contains and the hazards they may present.

Batteries must be discharged prior to disassembly. When removing cells from a battery pack and cutting open the battery cell case, it is possible to accidentally create a short. X-ray imaging allows the technician to see exactly where to cut. This minimizes the risk of cutting in the wrong place and creating an electrical short. Care must be taken not to crack the electrodes while unrolling the layers of material within the cell. Moisture and oxygen levels also need to be controlled when transporting disassembled cells to testing instruments, which may require keeping the cell in a vacuum-sealed container.
In addition to safety concerns, improper disassembly procedures carry the risk of misleading results. For example, shorts or contaminants introduced during disassembly may be attributed to the as-received failed battery. Personnel must be trained in proper disassembly procedures and have access to required tools and equipment, something that may not be feasible when failure analysis is done in-house.
Contracting Out Qualification and Testing
Most application engineers do not have a full suite of appropriate characterization tools at their disposal. These are expensive, specialized instruments that are usually not worth the investment unless the facility does extensive internal R&D. For most customers, it will be more cost-effective to contract out battery qualification and testing to a third-party independent testing facility that has the instruments and knowledge to conduct a thorough analysis. Independent facilities provide expert, unbiased analysis that battery manufacturers as well as their customers can trust.
CONCLUSION
Lithium-ion batteries are currently state of the art in terms of energy density per unit weight, which is leading to their increased use in products from mobile phones to electric and hybrid vehicles. However, lithium-ion batteries can also suffer catastrophic failure, putting a spotlight on the need for thorough qualification and testing throughout the supply chain.
Hiring an independent testing facility to conduct characterization on failed batteries provides objective, reliable results that OEMs can use to demonstrate why the battery failed and understand whether a defective battery is the source of a failure in their product, or whether they need to look elsewhere to determine the cause. Independent characterization is also valuable during the product development phase when evaluating batteries from multiple suppliers.
REFERENCES
- “Tesla Gigafactory.” Tesla. www.tesla.com/gigafactory. Web. Accessed Sept. 26, 2016.
- “Cars.” Plugincars. http://www.plugincars.com/cars. Web. Accessed Oct. 4, 2016.
- M. Karkafiris, “This Is Why Toyota Offers Two Different Battery Options In The New Prius.” Car Scoops, Nov. 30, 2015. http://www.carscoops.com/2015/11/this-is-why-toyota-offers-two-different.html.
- E. Musk, “Tesla Adds Titanium Underbody Shield and Aluminum Deflector Plates to Model S.” Medium.com, March 28, 2014. https://medium.com/@teslamotors/tesla-adds-titanium-underbody-shield-and-aluminum-deflector-plates-to-model-s-544f35965a0d#.v3kkxea1j. Accessed Oct. 4, 2016.
- F. Lambert, “Tesla Model S catches on fire during a test drive in France.” Electrek, Aug. 15, 2016. https://electrek.co/2016/08/15/tesla-model-s-catches-fire-test-drive-france/. Web. Accessed Oct. 4, 2016.
- F. Lambert, “Tesla driver dies in a Model S after hitting a tree, battery caught fire, Tesla launches an investigation.” Electrek, Sept. 7, 2016. https://electrek.co/2016/09/07/tesla-driver-dies-burning-model-s-hitting-tree-tesla-investigation/. Web. Accessed Oct. 4, 2016.
- S. LeVine, “The man who brought us the lithium-ion battery …” Quartz, Feb. 5, 2015. https://qz.com/338767/the-man-who-brought-us-the-lithium-ion-battery-at-57-has-an-idea-for-a-new-one-at-92/ Web. Accessed Oct. 7, 2016.
- A. Gozdz, C. Schmutz, J. Tarascon, “Rechargeable lithium intercalation battery with hybrid polymeric electrolyte.” U.S. Patent No. 5,293,618, issued March 22, 1994
- A. Gozdz, C. Schmutz, J. Tarascon, P. Warren, “Rechargeable lithium battery construction.” U.S. Patent No. 5,478,668, issued Dec. 26, 1995.
- J. Gelb, D. Finegan, D. Brett, P. Shearing. “Batteries Under the Microscope: 3D and 4D Image-Based Characterization” [abstract]. In: ECS Meeting Abstracts, 229th ECS Meeting; 2016; May 29 – June 2, San Diego. The Electrochemical Society; MA2016-01; Abstract no. 24.
- R. Sacci, et al., “Tracking Electrochemical Processes at the Nanoscale: Operando Transmission Electron Microscopy of Lithium Dendrite Formation” [Abstract]. In: ECS Meeting Abstracts, 229th ECS Meeting; 2016; May 29 – June 2, San Diego. The Electrochemical Society; MA2016-01; Abstract no. 212.
- J. Alvarado, et al., “Understanding the Factors That Improve the Cycling Performance of Silicon Anode for Li-Ion Batteries” [Abstract]. In: ECS Meeting Abstracts, 229th ECS Meeting; 2016; May 29 – June 2, San Diego. The Electrochemical Society; MA2016-01; Abstract no. 93.
- C. Wu, Y. Bai, F. Wu, “Fourier-transform infrared spectroscopic studies on the solid electrolyte interphase formed on Li-doped spinel Li1.05Mn1.96O4 cathode.” J. Power Sources 189(1), 89–94 (2009).
- S. Russell, A. Cresce, K. Xu, “Electrolyte-Electrode Interactions and Interphases” [Abstract]. In: ECS Meeting Abstracts, 229th ECS Meeting; 2016; May 29 – June 2, San Diego. The Electrochemical Society; MA2016-01; Abstract no. 303.
- N. Willard, B. Sood, M. Osterman, M. Pecht, “Disassembly Methodology for Conducting Failure Analysis on Lithium-ion Batteries.” J. Mater Sci: Mater Electron 22:1616 (2011). DOI 10.1007/s10854-011-0452-4
Would you like to learn more about improving performance of Lithium-Ion Batteries?
Contact us today to improve the performance of Lithium-Ion Batteries. Please complete the form below to have an EAG expert contact you.
