Optical Emission Spectroscopy for Composition Analysis of Complex Oxides
Home » Optical Emission Spectroscopy for Composition Analysis of Complex Oxides
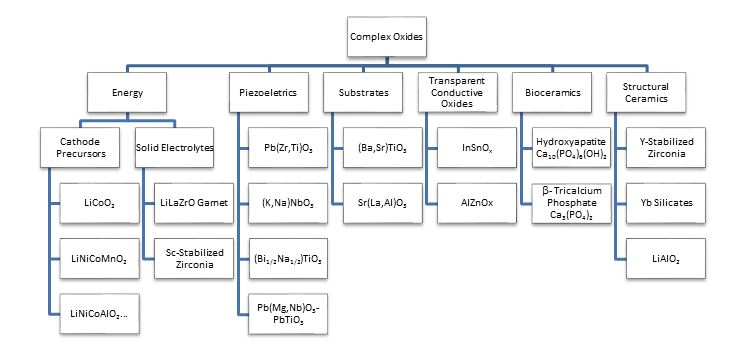
Complex oxides play increasingly important roles in advanced energy storage/harvesting, catalysis, sensor/actuation, optics, epitaxy substrates, electronics, bioceramics, structural ceramics, etc.2 Scheme 1 lists a number of complex oxides of industrial significance.
The multi-metal oxide nature increases the design flexibility for material property engineering. Complex oxide-related crystals greatly expand the substrate family from the commonly used III-V, II-IV and V family crystals for epitaxy growth. Perovskite (ABO3) substrates with continuous, tunable crystal lattice size is attainable in the range from 3.66 Å (LuAlO3 crystal) to 4.18 Å (LaLuO3 crystal) by employing elements of different ionic radii, including Al3+, Ga3+, Sc3+, Y3+, Lu3+, etc., as basic construction units.3
Defects engineering by doping also becomes an important means for tuning properties of complex oxides. In an effort to address cycling stability of lithium ion batteries, scientists from Argonne National Laboratory discovered doping a few percentages of Li in excess to lithium transition metal oxides (LTMO) can dramatically improve the long-term stability, which subsequently revolutionized the lithium ion battery industry.4
Whether on growing single crystals, textured ceramics or on defects engineering of complex oxides, maintaining uniform chemical composition and free of contamination is the first step toward product reliability and consistency. In addition, many properties are sensitive to the compositional fluctuation, thus demanding the knowledge of accurate composition to tighten the structure/property correlation. On the other hand, multi-metal oxide systems can seriously complicate material synthesis and processing. The interplay of differences in vapor pressure, solubility, diffusion rate, and the bonding chemistry of each component, increases the difficulty exponentially in controlling the final structure into a narrow concentration range during high temperature sintering. Achieving structure uniformity in scaling up production, including composition, has been key to turn a lab experiment into a viable technology.5
Eurofins EAG provides a total solution to address the R&D and production needs of complex oxide industry. We employ both solution-based and solid sampling techniques for accurate composition analysis:
- Our solid sampling techniques feature Laser Ablation – Inductively Coupled Plasma Mass Spectroscopy (LA-ICP-MS) and Wave Dispersive X-Ray Fluorescence Spectroscopy (WDXRF). Both techniques can achieve precision of 0.5 – 1% routinely.6 When calibrated with strict matrix-matching reference materials, relative accuracy (i.e., ratio of absolute error to “True” value) less than 1% can be readily achieved for major elements.
- Our solution technique features HP ICP-OES. The technique offers NIST-traceable results, irrespective of sample matrices. The accuracy, expressed as expanded uncertainty U, is typically on the order of 0.3 – 1% at approximately 95% confidence level, which is about an order of magnitude better than the classical ICP-OES.
HP ICP-OES Measurement Principle
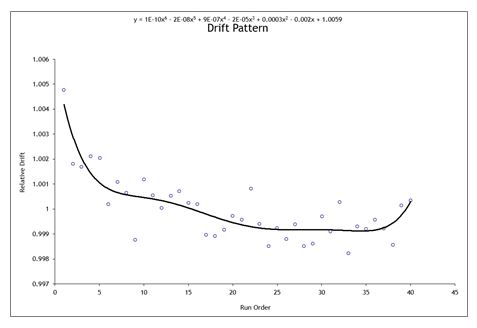
HP ICP-OES is a well-established enhancement of classical ICP-OES analytical procedures. It was originally developed at NIST as a means to determine the concentration of single element aqueous Standard Reference Materials (SRM).7 ICP-OES was developed under the assumption that it is sufficiently immune to matrix effects and instrument response nonlinearity. A key enabler of HP ICP-OES is the invention of a drift correction procedure to address what was the major source of uncertainty observed in classical ICP-OES results.8 An example of instrument drift is presented in Figure 1 for analysis of Li content in a LiMnO2 cathode precursor. During HP ICP-OES analysis, this instrument drift was applied to correct each measurement point with the fitted equation (shown in Figure 1).
As a relative method, HP ICP-OES establishes two ratios (which are ratios again) for quantification,
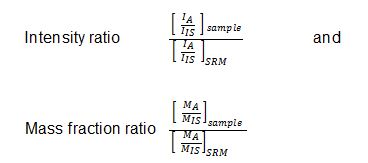
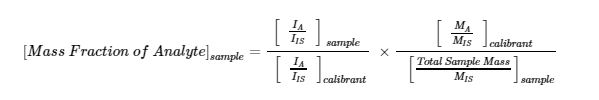
wherein A – analyte, IS – internal standard, SRM – standard reference material, I – signal intensity, M – total mass in gram. A calibration relationship is then established to infer the composition of the unknown samples from their measured signal ratios using the following equation,
EAG’s HP ICP-OES protocol incorporated the suggested improvement on the original NIST procedures to further reduce the uncertainty of measurement:9
- exactly matching the analyte mass fractions, internal standard mass fractions, and matrix compositions among the solutions prepared for an analysis;
- gravimetrically establishing mass fraction ratios;
- multiple replicates at 100 – 250 mg per replicate;
Uncertainty Estimation
The uncertainty of the HP ICP-OES measurement considered three major sources of errors: error from measuring SRM solutions USRM Meas , error from measuring unknown sample solutions USample Meas , and the error of the certified values of SRM USRM . The combined uncertainty Uc is calculated by
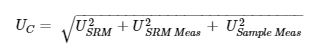
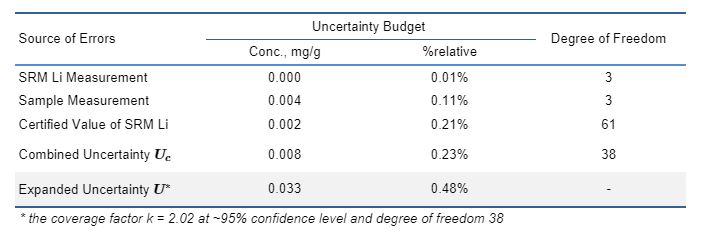
Table 1 illustrates the uncertainty budget estimation during HP ICP-OES analysis of a LiMnO2 sample. The combined uncertainty is 0.23%relative. The contribution from measuring SRM Li standard and sample are small (0.01%relative and 0.11%relative, respectively), indicating that the instrument drift issue is well controlled, and that compositional variation inherent to this particular sample is minor. The largest source of error is from the certified value of SRM (0.21%relative). For homogeneous samples, the limiting factor to further reduce measurement uncertainty relies on the availability of SRM with tighter uncertainty. The expanded uncertainty U is estimated by
wherein the coverage factor k is determined from the student’s t table based on the calculated degree of freedom of the analysis. The expanded uncertainty U ~ 0.45% was achieved for Li measurement (Table 1).
Advanced Sample Digestion Capability in EAG
HP ICP-OES analysis of complex oxides is not possible without the support of robust digestion techniques. Complex oxides, particularly after sintering, are more often than not chemically resistant to acid attack. Like any other dissolution, digestion of complex oxides involves two successive steps – dissolving the matrix and solvating the released ions. Many complex oxides regularly require an aggressive acid combination and heating to 200 – 250 °C under 40 – 80 bar pressure for long duration (10 – 20 hours) for complete digestion. The liberated metal ions must be immediately solvated, either by H2O molecules in the vicinity, by complexation with anionic ligands from the dissociated acids or by chelating molecules.
The challenge in full digestion of complex oxides is demonstrated by the (Sr,Ba)TiO3 sample system. An acid mixture of HF and HNO3 is commonly used to dissolve the titanate framework via microwave acid digestion. To keep Sr and Ba ions in the solution, the F- ion concentration in the acid mixture must be properly balanced, e.g., with the addition of boric acid, such that formation of low solubility SrF2 (solubility product ksp=8×10-10) and BaF2 (ksp=1×10-6) is not kinetically favorable. Similar challenges are encountered in digesting complex oxides such as aluminates, silicates, zirconates and other titanates families containing alkaline earth ions (e.g., Mg2+, Ca2+, Sr2+, Ba2+) or rare earth ions (such as Sc3+, Y3+, La3+, Ce3+, etc.).
EAG deploys various advanced digestion techniques, including high pressure microwave, parr bomb, fusion and tube digestion. Our ICP team developed a vast array of chelating chemistry for high temperature digestion to ensure complete digestion of complex oxides into an acid solution suitable for HP ICP-OES analysis.
Case Studies
1. Lithium transition metal oxides (LTMO) cathode precursors
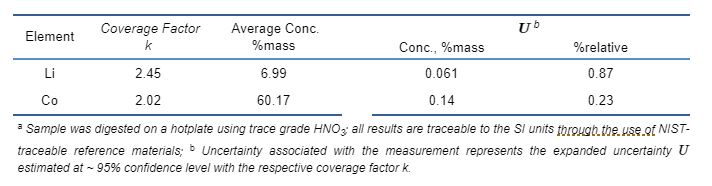
Layered Li-excess transition metal oxides are most important cathode precursors for lithium ion batteries. A proper excess amount of Li is known to stabilize the layer structure during the lithiation/de-lithiation cycle. Table 2 summarizes the HP ICP-OES result for a LiCoO2 sample purchased from Sigma-Aldrich. The expanded uncertainties of the measurement are U = 0.87%relative and 0.23%relative for Li and Co respectively, at ~95% confidence level. With such tight uncertainty measurement, it allows the accurate determination of Li/Co ratio. In this case, it is estimated there is 1.2%atom deficiency of Li in the investigated LiCoO2. HP ICP-OES technique has been used to accurately assess the Li/TM ratios and the qualification of many Li-excess LTMO precursors, including LiNiCoAlO2, LiNiCoMnO2, Li2RuO3, Li2IrO3, etc.
2. Bioceramic precursors
Hydroxyapatite, Ca10(PO4)6(OH)2, is the primary mineral component of bone and teeth to impart hardness and strength. Structural characterization requirement of hydroxyapatite and Beta-tricalcium phosphate coatings and powders has been specified in FDA guidance document 510(K) and various ASTM methods10. As bioceramic precursors, the stoichiometric Ca/P ratio of hydroxyapatite is 1.67, which leads to an overall of good mechanical properties in terms of hardness and fracture toughness. In practice, large variation in composition exists. As a matter of fact, hydroxyapatite can be a highly non-stoichiometric solid.
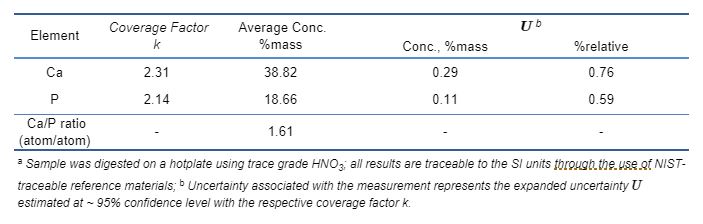
Table 3 illustrates the composition analysis of one commercial lot of hydroxyapatite (Sigma-Aldrich) using HP ICP-OES method. The expanded uncertainties for Ca and P measurement are both within 1%relative, at ~ 95% confidence level. Such tight uncertainty of measurement enables rigorous composition check of Ca/P ratios of incoming hydroxyapatite and β-tricalcium phosphate feedstocks. In this case, the determined Ca/P ratio is 1.61, indicative of Ca-deficiency of this particular lot of hydroxyapatite.
Summary
Eurofins EAG offers solid sampling such as LA-ICP-MS and WDXRF for high precision composition analysis of solid samples. Alternatively, we offer solution sampling technique ICP-OES for composition analysis of complex oxides when matrix standards are not readily available. As an enhancement to classical ICP-OES, HP ICP-OES method reduces the measurement uncertainty by drift correction, calibrating using intensity ratios and mass fraction ratios, etc. In this paper, we demonstrated that HP ICP-OES can be used to determine multiple elemental concentrations simultaneously for complex oxides such as those listed in Scheme 1, when coupled with advanced digestion capability. All results in HP ICP-OES measurement are traceable to NIST standards, with expanded uncertainties on the order of 0.3 – 1.0%, which are about an order of magnitude better than the classical ICP-OES.
Aside from complex oxides, Eurofins EAG’s HP ICP-OES analytical service has been widely used in production control and certification of advanced alloys, sputtering targets, phase change memory chalcogenides, etc.
Footnotes
- “Complex Oxides: An Introduction”. Editors: Thomas Vogt, and Douglas J. Buttrey. World Scientific Publishing Company Pte Limited, 2019. ISBN: 9813278579, 9789813278578.
- Ueckel et al. J Crystal Growth, 2017, 457, 137;
- Johnson et al., Electrochem. Commun., 6, 1085 (2004); Thackeray et al., J Mater Chem, 2007,17, 3112
- Penn State Applied Research Lab launched a program with Navy to scale up the production of texture ceramic addressing the manufacturing consistency issues.
- Strossman, G., “Alloy Characterization Using Wave Dispersive X-Ray Fluorescence Spectroscopy (WDXRF)”. EAG Application Notes.
- Salit et al. Anal Chem, 2001, 73, 4821.
- Salit et al. Anal Chem, 1998, 70, 3184.
- Winchester et al. Anal Chem, 2010, 82, 187675.
- a) FDA 510(K) Information Needed for Hydroxyapatite Coated Orthopedic Implants; b) ASTM F1185 – 03(2014): Standard Specification for Composition of Hydroxyapatite for Surgical Implants; c) ASTM F1088 – 18: Standard Specification for Beta-Tricalcium Phosphate for Surgical Implantation.
Would you like to learn more about Composition Analysis of Complex Oxides?
Contact us today for your HP ICP-OES for composition analysis of complex oxide needs. Please complete the form below to have an EAG expert contact you.