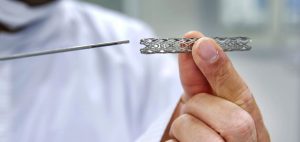
Assessing Nickel Leaching on Cardiovascular Stents
EAG evaluate corrosion and leaching of nickel-rich implanted medical devices including nitinol, stainless steel and MP35N.
For medical devices and biomaterial companies, EAG Laboratories offers the most diverse and comprehensive suite of testing and engineering support available. EAG Laboratories has established itself within the technology and biomedical space by developing scientific expertise in difficult analytical measurements, characterizations of complex materials and systems, and a pioneering approach to problem-solving.
Medical device and diagnostics companies continue to develop newer technologies to provide better treatments, less discomfort for the patient and improved patient assessment. Continued advances in technology, chemistry and materials are integral to the future progress of medical devices and diagnostics. In the near future, medical device and diagnostics companies are faced with developing new platforms with aggressive requirements on chemistries, materials and components. The devices and diagnostics of tomorrow will require smaller features, thinner coatings and improved stability while improving effectiveness and safety.
With laboratories located around the world, EAG scientists have supported customers creating products for a wide range of medical devices such as ultrasounds, surgical tools, vascular devices, stents, dental and orthopedic implants, catheters and medical robotics.
Turn to EAG. WE KNOW HOW.
EAG offers a comprehensive suite of services for the medical device industry, meeting the industry’s exacting standards for tight controls of material properties, composition and chemistry. Here are a few ways we can help:
Our extractables and leachable (E&L) testing programs to help clients confirm the safety of their products, as well as confirming material consistency and quality.
EAG is the premier scientific services company with expertise in biomedical products, chemistries and materials, regulatory insight, and a proper sense of urgency to deliver meaningful results with an impact.
As industry leaders in deformulation (reverse engineering), our scientists can characterize the composition of medical plastics, determining raw materials and additives.
Evaluating pitting corrosion and characterizing surface oxides can help assess the safety of nickel-containing implantable medical devices.


MDR guidelines require collection of data and documentation that medical devices do not contain carcinogens, mutagens, or reproductive toxic substances.
An improperly treated surface may be harmful to the patient, and depending on these characteristics could trigger an allergic response, bacterial infection, or even a blot clot.
Particle analysis and identification can help determine the root cause of a product problem and evaluate corrective actions.
We have the engineering expertise to design, develop, test, analyze and debug using ATE, Burn-in, ESD and latch-up testing, system-level testing, reliability, warpage analysis and IC-level failure analysis.
We investigate fractures, fatigue, corrosion, contamination, wear, heat, stress-related failures and abrasion concerns.
Inspection of raw materials during key phases of manufacturing can aid in the identification and elimination of unwanted impurities in final products, as well as to evaluate a new vendor or equivalent material selection.
During the concept, research and early design phase of product development, we provide competitive product insight, assess the chemistries of a material and perform proof-of-concept studies.
During the design and development phase, we support engineers that are evaluating materials, performing preliminary performance testing and developing future safety testing strategies.
EAG offers extractables & leachables, ISO 10993 Part 18 testing and contamination control to support a successful submission to the FDA and to establish an efficient and effective manufacturing process.
Once the device is approved and in the market, EAG provides continued support to evaluate quality issues, control manufacturing, manage supply chains and investigate field failures to determine the root cause.
EAG’s intellectual property experts offer litigation support for patent infringement challenges related to products claims, as well as help companies confront potential threats to their valuable intellectual property.
EAG’s product liability experts bring years of experience investigating failures, providing scientific and engineering expertise, data interpretation and expert testimony for hundreds of product liability and insurance claims

We understand the need for first-to-market breakthrough technologies and EAG can collaborate to provide results-driven solutions to the most complex of analytical investigations. Our experienced experts will work with your team to troubleshoot and choose the right material for your application or improve a manufacturing process.
Contact us today for your medical device testing needs. Please complete the form below to have an EAG expert contact you.

EAG evaluate corrosion and leaching of nickel-rich implanted medical devices including nitinol, stainless steel and MP35N.

A device manufacturer observed defects in a lot of thermoplastic tubing. The tubing exhibited signs chemical attack or exposure to heating.
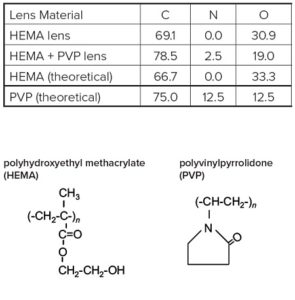
EAG Laboratories helps you understand contact lenses surface chemistry, critical to optimizing design and engineering optimal performance.
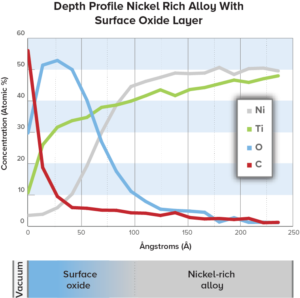
Nickel biocompatibility assessments of intravascular stents from EAG includes evaluating corrosion, surfaces and nickel ion release testing.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.