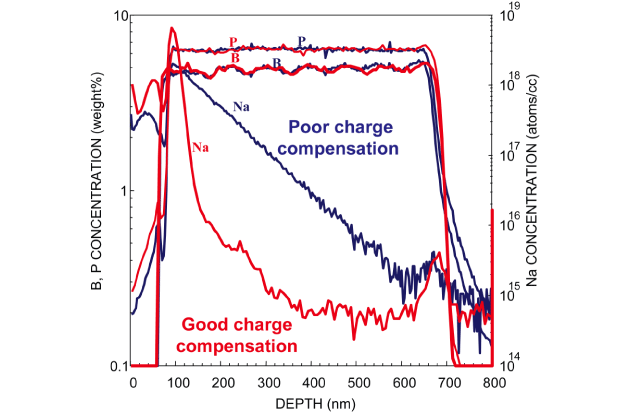
Impurity depth direction analysis of various glass materials by SIMS
SIMS analysis is used to measure trace impurities in various materials such as semiconductors, metal, and insulating materials
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.