
Glass Analysis Webinar
In the full webinar we will focus on Glass Analysis looking at Chemical and Physical Measurements to address manufacturing issues
Home » Failure Analysis of Medical Device Electronics
Medical device and diagnostics companies have taken great strides in the development of newer technologies to provide better patient assessment and treatments. Devices of the future will require smaller features, lighter materials, and improved stability. Not only is it important to assess the materials and chemistry in medical devices, but it is also equally as important to assess medical device electronics to ensure safety and reliability. From hearing aids to heart pacemakers to catheters and more, advanced electronics can be seen in many of today’s medical devices.
Every day the medical industry lunges forward with more advanced instruments and higher quality consumer products. This new level of sophistication also translates into more complex R&D processes. As the manufacturing process gets more complex, there are many more opportunities for things to go awry. Identifying, diagnosing, and remedying failures becomes dramatically more challenging. Our experienced engineers and scientists possess a comprehensive array of advanced tools and techniques that can perform failure analysis on a large range of electronic devices.
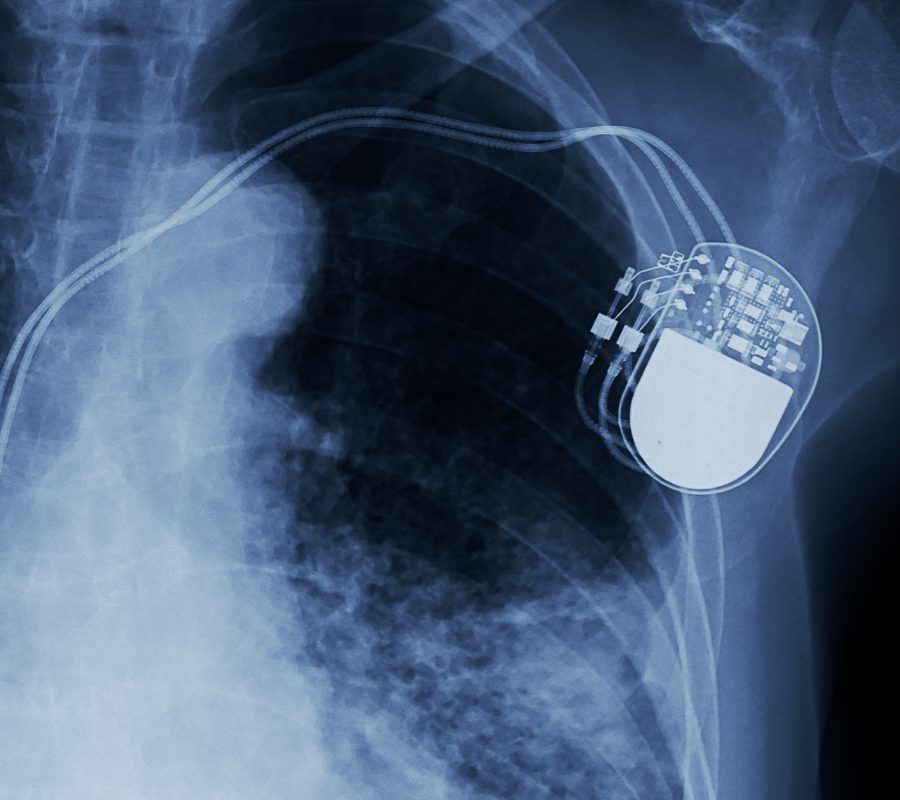

Eurofins EAG Laboratories offers the most diverse and comprehensive suite of testing and engineering support available for medical devices. With failure analysis services that range from non-destructive examination to physical analysis for root cause identification, our failure analysis team has the engineering expertise to help solve problems at any phase of the product cycle. EAG understands the stringent regulations, requirements and expectations of medical devices and are therefore the perfect partners to assist with advancements in the medical device industry.

In the full webinar we will focus on Glass Analysis looking at Chemical and Physical Measurements to address manufacturing issues

For over 40 years, EAG has been involved in the entire glass value chain, from raw starting materials to final products.
ICP-MS is a multi-elemental bulk chemical analysis technique that can determine simultaneously up to 70 elements in a single sample.
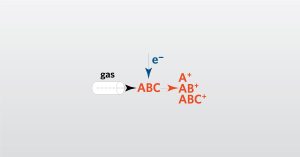
In this webinar we will focus on Gas Chromatography-Mass Spectrometry (GC-MS) which allows for the identification of specific molecules
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.