Advances in Materials Characterization of Medical Device Webinar
In this webinar we introduce EAG Laboratories advances in Materials and Surfaces Characterization of Medical Devices
Home » Medical Device

In this webinar we introduce EAG Laboratories advances in Materials and Surfaces Characterization of Medical Devices
In this webinar we introduce how a 3rd Party Analytical Lab Can Help Support Your Company’s Compliance with EU’s MDR

June 27, 2024
During this live Ask the Expert event, we will answer pre-submitted questions from our audience regarding Elemental Analysis with Inductively Coupled Plasma (ICP-OES, ICP-MS, LA-ICP-MS). These techniques provide full survey major, minor, trace element analysis, and purity certification for up to 69 measurable elements.

In vitro biocompatibility testing is a powerful tool in the risk assessment of wearable devices. View our webinar to learn more!
EAG evaluate corrosion and leaching of nickel-rich implanted medical devices including nitinol, stainless steel and MP35N.
Nickel biocompatibility assessments of intravascular stents from EAG includes evaluating corrosion, surfaces and nickel ion release testing.

October 2-4, 2024
Eurofins EAG Laboratories will present at the 34th Annual BioInterface Workshop & Symposium.
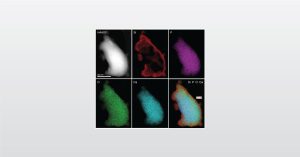
Biomedical Morphology and Microstructure through imaging analysis – characterizing crystallographic phase and producing elemental maps.
Biomedical surface analysis looks at properties critical to medical and pharmaceutical products, and offer answers to difficult problems.
Catheter and balloon analysis for medical device development support includes deformulation, contaminant identification and failure analysis.
In this webinar we introduce Characterization of Bioceramics for Surgical Implants using analytical tools for full qualification
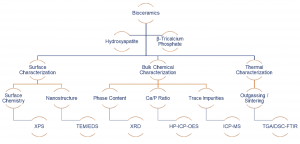
This paper will demonstrate how analytical tools can be used for the quality control of hydroxyapatite and β-tricalcium phosphate powders

Stents characterization from EAG includes nitinol, stainless steel and MP35N. Experiences with fatigue resistance and hydrogen embrittlement.

In this webinar we introduce Chemical Compatibility of Polymers in Medical Devices and prevent material problems in the field
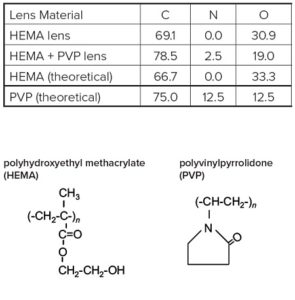
EAG Laboratories helps you understand contact lenses surface chemistry, critical to optimizing design and engineering optimal performance.

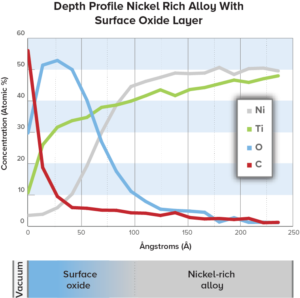
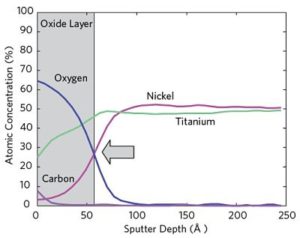
Medical device corrosion resistance optimized by using Auger depth profile & oxide layer thickness for device surface characterization.

This webinar covers FDA regulation of medical devices, the most commonly used test standards, and custom testing at Eurofins EAG Laboratories.

An injection molded component in a consumer product was found to have an increased failure rate over a three-month period.

A client requested assistance to investigate a recurrent issue with the curing failure of a silicone adhesive joint between metal components.

A client wanted to investigate the delamination of food packaging multilayer laminate. Roll stock did not meet seal strength specification.

May 15, 2024
Check out our online symposium presented by EAG Laboratories and Exponent!
EAG Laboratories offer comprehensive testing for materials, electronics and drugs for medical offerings

6th May 2021
EAG Laboratories has expanded its medical device testing capabilities with a new laboratory located in St. Louis, Mo.

13th December 2022
Manufacturing Technology Insights recently named EAG Laboratories a Top 10 Corrosion Services Provider. The publication featured an interview with Dana Medlin, our Vice President of Metallurgical Engineering.

August 28, 2023
Manufacturing Technology Insights named Eurofins EAG Laboratories a Top 10 Corrosion Services Provider for the second year in a row.

17th November 2022
EAG has been approved as a suitable commercial laboratory to provide MIL-STD 883/750 Test Method 1018 Internal Water Vapor Content Testing for hermetically sealed electronic devices.
8th June 2023
Now, with RGA included in the scope of ISO 17025, RGA testing at EAG is a premium choice to perform gas analysis.

The roughness of a surface and how it interacts with surrounding materials and elements can have a significant impact on material technology and its functionality.

XRF is a non-destructive technique that is used to quantify the elemental composition of materials.

A company was investigating environmental stress cracking of polycarbonate components used in a conveyor device that was sanitized.

Essentials of Particulate Testing: USP <788> and AAMI TIR42 While there are many standards available for particulate testing, USP <788> and AAMI TIR42 are the

Eurofins EAG has a wide array of analytical techniques to verify the cleanliness of medical devices. We’ve helped our customers evaluate the cleanliness of their products using techniques including NVR analysis, often in combination with FTIR, XPS and TOF-SIMS.

All materials have trapped gasses inside. Learn how EAG utilizes Evolved Gas Analysis to analyze what gasses are being released.
Exclusion Testing for Material Compliance Regulations of Medical Device Components has multiple regulating bodies.

Through database building, EAG provides higher-quality chemical characterization reports with reduced turnaround times to support medical device biocompatibility programs.

November 13-14, 2024
Eurofins EAG Laboratories will present at Extractables & Leachables West 2024.

EAG offers advanced expertise and a risk-based approach for medical device extractable study design and execution, including the latest FDA expectations.

As medical devices become more complex, there are many more opportunities for things to go awry. Identifying, diagnosing, and remedying failures becomes dramatically more challenging. We can help.

The analytical evaluation threshold (AET), an important part of the extractables and leachables testing in these studies, needs to be calculated for each instrumental technique.

Biocompatibility assessments are a battery of tests performed to evaluate a product, medical device, or other material for the risk of biological hazards.

EAG Laboratories pursues quality in all aspects of our work for customers. ISO certifications and ongoing audits serves many market sectors.

FTIR and Raman are spectroscopy techniques that provide molecular information from various types of materials.
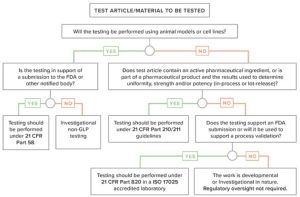
Medical device regulations are not entirely clear, EAG shows the decision process for contract analytical laboratories to support development.
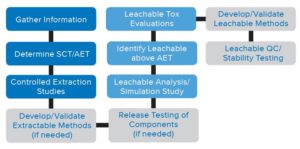
E&L methods pose challenges as they transition from R&D environment to routine QC testing. Understanding these challenges will help you avoid costly delays.

In Vitro Diagnostics test and system development support from the materials sciences experts at EAG Laboratories, contact us today!

A device manufacturer observed defects in a lot of thermoplastic tubing. The tubing exhibited signs chemical attack or exposure to heating.

Surface cleanliness is critical in how a material or a layer on a product interacts with other surfaces, which in turn can affect its functionality.
Respiratory protection testing, cleaning compatibility, sanitizing liquids and wipes, supply chain and manufacturing quality, failure analysis

December 12, 2023
Check out our online symposium presented by EAG Laboratories and Gradient!

February 4-6, 2025
Eurofins EAG Laboratories will exhibit and present at MD&M West in Anaheim. Dr. Philip Ferko, Senior Scientist with EAG, will present in the MD&M West Center Stage at 12pm on February 4.

September 25-26, 2024
Eurofins EAG Laboratories will exhibit and co=present with Gradient at MEDevice Boston 2024.

Drive productivity while keeping pace with evolving regulations
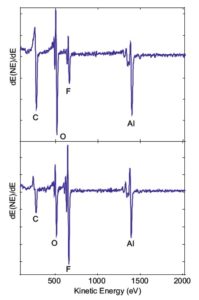
Auger Electron Spectroscopy applied to electronic circuitry problems involving the integrity of wire or ball bonding to metallic bond pads.

Ensuring medical devices are safe, compliant and effective

Device concept and medical device researchers need materials answers quickly so the can proceed with device development. Ask EAG!

Cracks were observed in transparent thermoformed plastic packaging during visual quality checks. Failures occured in specific material lots.

September 11, 2024
Eurofins EAG Laboratories is the presenting sponsor for the North American Biocompatibility Summit (NABS) at Quincy Hall in Minneapolis, Minnesota.

Analytical testing to support changes in raw materials and processing conditions used in manufacturing memory foam, as well as odor

Orthopedic implants composed of metallurgical alloys, plastics and critical surface chemistries require characterization

Part 18: Chemical characterization of materials, part of ISO 10993 pertains to evaluating biocompatibility and safety of medical devices.

Blisters were observed in paint applied to steel plates that had undergone treatment with a corrosion inhibitor. An investigation was performed to determine if there was evidence of contamination on the inside of the blister.
In Vitro testing uses cell-based biological models instead of animals or humans.
To be hermetically sealed essentially means to be airtight so that nothing can come in or get out (i.e., gas, moisture, liquid, etc.).
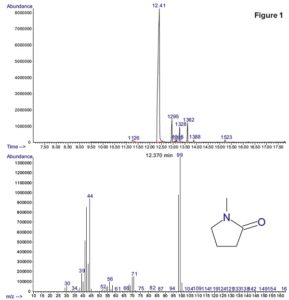
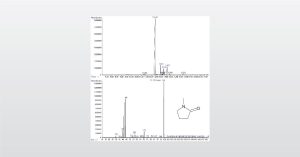
Detecting Levels of residual solvent concentrations in medical devices with Dynamic headspace Gas Chromatography-Mass Spectromety (GC-MS)
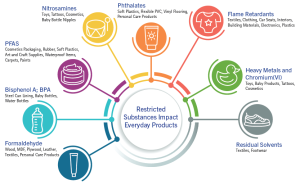
As the list of restricted substances grows, testing demands customized methods to identify issues early. This is a complex issue that requires a strategic approach.
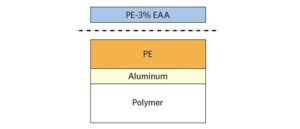
XPS and TOF-SIMS to solve adhesive failure of a polyethylene-ethylene acrylic acid (co-polymer) heat seal to polyethylene in a medical device.

May 6-10, 2024
Eurofins EAG Laboratories will be presenting “Thermal Oxide Characterization – Heat Treat Modality vs. Composition“ on May 10th. We hope to see you there!
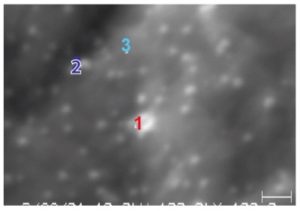
Auger Electron Spectroscopy (AES) is effective in determining the quality of passivation layers to prevent corrosion in medical devices.
Cold stage analysis has been shown to be effective for samples that are not solids at room temperature or that have components that are volatile in a vacuum system, such as the antibacterial coated sutures.

Analytical methodology to evaluate a variety of different face masks for a class of chemicals known as volatile organic compounds
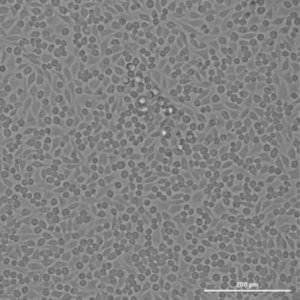
In the world of medical device safety testing, ISO 10993-5 plays a crucial role. A key aspect of this, which is sometimes overlooked in certain studies, is the T0 time point, or time zero.
LA-ICP-MS is a technique that uses direct micro-scale sampling to provide high precision elemental characterization of solid materials.

Polymeric films used in an outdoor application experienced reduced adhesion over time. The client suspected that UV degradation was occurring.

In our interconnected and changing world, it is important to know how to respond to potential electronic system failures.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.